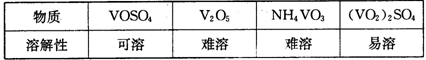
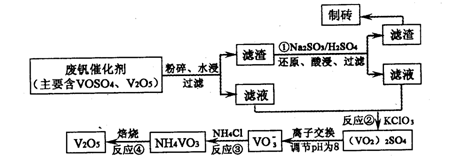
��Ŀ����
��ˮ��ȡ֮�����Ļ���ԭ����Դ�⣬�Ӻ�ˮ�п���ȡ���ֻ���ԭ�ϡ������ǹ�ҵ�϶Ժ�ˮ�ļ����ۺ����õ�ʾ��ͼ��
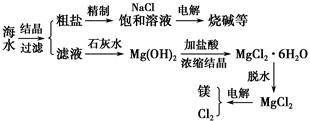
�Իش��������⣺
��1�������к���Ca2����Mg2����SO42-�����ʣ�����ʱ�����Լ�Ϊ��A.���B.BaCl2��Һ��C.NaOH��Һ��D.Na2CO3��Һ�������Լ���˳����________________________________________________________________��
��2����ⱥ��ʳ��ˮʱ�����Դ���������ĵ缫�Ϸ����ķ�ӦΪ________�����Դ���������ĵ缫������ҺpH________���������䡱��С��������1 mol���ӵĵ���Ϊ96 500 C�����õ���ǿ��Ϊ100 A���Ⱥ�����������3��13�룬���������Ϲ����ռ�������________mL��STP��������֤����ʳ��ˮ��Ũ�Ȳ��䣬��ɲ�ȡ�ķ�����________________________________________________________________��
��3����MgCl2��6H2O������ˮ����ˮMgCl2ʱ��MgCl2��6H2O������________�����м�����ˮ���������������_____________________________________________________��
��4�������ˮMgCl2���õ�þ��������������________��������ȴ��
A��H2 B��N2 C��CO2 D��O2

�Իش��������⣺
��1�������к���Ca2����Mg2����SO42-�����ʣ�����ʱ�����Լ�Ϊ��A.���B.BaCl2��Һ��C.NaOH��Һ��D.Na2CO3��Һ�������Լ���˳����________________________________________________________________��
��2����ⱥ��ʳ��ˮʱ�����Դ���������ĵ缫�Ϸ����ķ�ӦΪ________�����Դ���������ĵ缫������ҺpH________���������䡱��С��������1 mol���ӵĵ���Ϊ96 500 C�����õ���ǿ��Ϊ100 A���Ⱥ�����������3��13�룬���������Ϲ����ռ�������________mL��STP��������֤����ʳ��ˮ��Ũ�Ȳ��䣬��ɲ�ȡ�ķ�����________________________________________________________________��
��3����MgCl2��6H2O������ˮ����ˮMgCl2ʱ��MgCl2��6H2O������________�����м�����ˮ���������������_____________________________________________________��
��4�������ˮMgCl2���õ�þ��������������________��������ȴ��
A��H2 B��N2 C��CO2 D��O2
��1��BCDA��CBDA����2��2Cl����2e��=Cl2�������4 480������Һ��ͨ��һ������HCl����
��3��HCl������Mg2��ˮ�⡡��4��A
��3��HCl������Mg2��ˮ�⡡��4��A
��1����С�������ӳ����⡣����ԭ�����ڳ�ȥCa2����Mg2����SO42-ʱ�����ܴ����������ӡ����ԣ������Ĺؼ��ǰ��պü������ӵ�˳��Ba2��������CO32-֮ǰ���룻��CO32-��OH��������H��֮ǰ���룬����B��C�����Ⱥ�D��A�����ȷ�ǰ���ֱ������B��C֮���������������
��2����ⱥ��ʳ��ˮʱ�����������������������ķ�Ӧ�ǣ�2Cl����2e��=Cl2�����븺�����������������ķ�Ӧ�ǣ�2H����2e��=H2����H���������ģ�ʹ����Һ��c��OH��������pH���������ݵ���Cl2��H2������Ҫȷ��ԭ��ҺŨ�Ȳ��䣬ֻ������ϵ��ͨ��һ������HCl�����Բ�����ʧ���⡢�ȡ��״����Ǽ������ᣬʹ��ҺŨ�ȱ�С��
��3������ˮ��ƽ��MgCl2��H2O Mg��OH��Cl��HCl�����ƶ���
Mg��OH��Cl��HCl�����ƶ���
��4��þ���������뵪���������Ͷ�����̼��Ӧ������ֻ������������ȴ��
��2����ⱥ��ʳ��ˮʱ�����������������������ķ�Ӧ�ǣ�2Cl����2e��=Cl2�����븺�����������������ķ�Ӧ�ǣ�2H����2e��=H2����H���������ģ�ʹ����Һ��c��OH��������pH���������ݵ���Cl2��H2������Ҫȷ��ԭ��ҺŨ�Ȳ��䣬ֻ������ϵ��ͨ��һ������HCl�����Բ�����ʧ���⡢�ȡ��״����Ǽ������ᣬʹ��ҺŨ�ȱ�С��
��3������ˮ��ƽ��MgCl2��H2O
 Mg��OH��Cl��HCl�����ƶ���
Mg��OH��Cl��HCl�����ƶ�����4��þ���������뵪���������Ͷ�����̼��Ӧ������ֻ������������ȴ��

��ϰ��ϵ�д�
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
�����Ŀ
 2HCl
2HCl 2NaOH��H2����Cl2��
2NaOH��H2����Cl2��