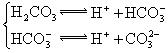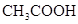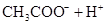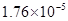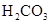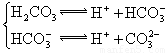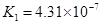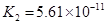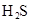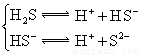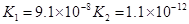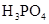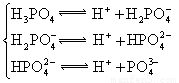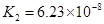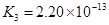��Ŀ����
��������,��һ���¶��´ﵽ����ƽ��ʱ,������Ũ�ȴ���һ�ֶ����Ĺ�ϵ����25��ʱ��HA![]() H++A�D����K=
H++A�D����K=![]() ��ʽ�У�KΪ����ƽ�ⳣ����ֻ���¶��йأ�cΪ������ƽ��Ũ�ȡ��±��Ǽ��ֳ�������ĵ���ƽ�ⳣ����25�棩��
��ʽ�У�KΪ����ƽ�ⳣ����ֻ���¶��йأ�cΪ������ƽ��Ũ�ȡ��±��Ǽ��ֳ�������ĵ���ƽ�ⳣ����25�棩��
�ش����и��⣺
��1��Kֻ���¶��йأ����¶�����ʱ��Kֵ__________�����������С���������䡱����
��2�����¶���ͬʱ���������Kֵ��ͬ����ôKֵ�Ĵ�С�����Ե����ǿ���кι� ϵ?_____________________________��
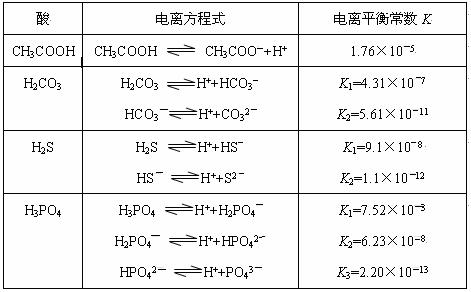
��3������CH3COOH��H2CO3��HCO3����H2S��HS����H3PO4��H2PO4����HPO42�����������ᣬ����������ǿ����_______����������_____________��
��4����Ԫ�����Ƿֲ�����ģ�ÿһ��������Ӧ�ĵ���ƽ�ⳣ��������ͬһ�ֶ�Ԫ�����K1��K2��K3֮������������ϵĹ��ɣ��˹�����________________�������˹��ɵ�ԭ����___________________________________��
��1���¶����ߣ��ٽ����룬����Kֵ����
��2��KֵԽ�������������Ũ��Խ����������Խǿ
��3��H3PO4 HPO42�D
��4��K1��K2��K3��1��10�D5��10�D10 ��һ�����������H+����һ����������������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д��������ᣬ��һ���¶��´ﵽ����ƽ��ʱ��������Ũ�ȴ���һ�ֶ����Ĺ�ϵ���±���25��ʱ���ֳ�������ĵ���ƽ�ⳣ��
| �� | ���뷽��ʽ | ����ƽ�ⳣ��K |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
�������ᣬ��һ���¶��´ﵽ����ƽ��ʱ��������Ũ�ȴ���һ�ֶ����Ĺ�ϵ���±���25��ʱ���ֳ�������ĵ���ƽ�ⳣ��
| �� | ���뷽��ʽ | ����ƽ�ⳣ��K |
| | | |
| | | |
| | | |
| | | |
�ش����и��ʣ�
��1��Kֻ���¶��йأ����¶�����ʱ��Kֵ________�����������С���������䡱����
��2�����¶���ͬʱ���������Kֵ��ͬ����ôKֵ�Ĵ�С�����Ե����ǿ���кι�ϵ?__________________��
��3������CH3COOH��H2CO3��HCO3-��H2S��HS-��H3PO4��H2PO4-��HPO42-���������ᣬ����������ǿ����_________����������________��
��4����Ԫ�����Ƿֲ�����ģ�ÿһ��������Ӧ�ĵ���ƽ�ⳣ��������ͬһ�ֶ�Ԫ�����K1��K2��K3֮������������ϵĹ��ɣ�����H3PO4�˹�����________________�������˹��ɵ�ԭ����_________________________��
![]() ��5������ƽ�ⳣ������ʵ��ķ����ⶨ�����ģ����Ѿ����ij�¶��� NH3∙H2O��Һ�д������·�Ӧ��NH3∙H2O NH4++OH- ��֪0.10 mol��L-1NH3∙H2O��Һ�У��ﵽƽ��ʱ��Cƽ����OH-��=4.2 �� 10-3mol��L-1��Cƽ����NH3∙H2O����C��ʼ��NH3∙H2O����ˮ�ĵ���ɺ��Բ���;
��5������ƽ�ⳣ������ʵ��ķ����ⶨ�����ģ����Ѿ����ij�¶��� NH3∙H2O��Һ�д������·�Ӧ��NH3∙H2O NH4++OH- ��֪0.10 mol��L-1NH3∙H2O��Һ�У��ﵽƽ��ʱ��Cƽ����OH-��=4.2 �� 10-3mol��L-1��Cƽ����NH3∙H2O����C��ʼ��NH3∙H2O����ˮ�ĵ���ɺ��Բ���;
����pH��ֽ������Һ��pHֵ���������Cƽ����OH-�����ⶨ��ҺpHֵ�IJ�����______________��
�ڲ���Cƽ����NH3∙H2O���ķ��������_____________����������ƣ�
������¶��¸÷�Ӧ��ƽ�ⳣ��K.(д��������̣�����������2λ��Ч����)
�������ᣬ��һ���¶��´ﵽ����ƽ��ʱ��������Ũ�ȴ���һ�ֶ����Ĺ�ϵ���±���25��ʱ���ֳ�������ĵ���ƽ�ⳣ��
|
�� |
���뷽��ʽ |
����ƽ�ⳣ��K |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
�ش����и��ʣ�
��1��Kֻ���¶��йأ����¶�����ʱ��Kֵ________�����������С���������䡱����
��2�����¶���ͬʱ���������Kֵ��ͬ����ôKֵ�Ĵ�С�����Ե����ǿ���кι�ϵ?__________________��
��3������CH3COOH��H2CO3��HCO3-��H2S��HS-��H3PO4��H2PO4-��HPO42-���������ᣬ����������ǿ����_________����������________��
��4����Ԫ�����Ƿֲ�����ģ�ÿһ��������Ӧ�ĵ���ƽ�ⳣ��������ͬһ�ֶ�Ԫ�����K1��K2��K3֮������������ϵĹ��ɣ�����H3PO4�˹�����________________�������˹��ɵ�ԭ����_________________________��
 ��5������ƽ�ⳣ������ʵ��ķ����ⶨ�����ģ����Ѿ����ij�¶��� NH3∙H2O��Һ�д������·�Ӧ��NH3∙H2O NH4++OH-
��֪0.10 mol��L-1
NH3∙H2O��Һ�У��ﵽƽ��ʱ��Cƽ����OH-��=4.2 �� 10-3mol��L-1��Cƽ����NH3∙H2O����C��ʼ��NH3∙H2O����ˮ�ĵ���ɺ��Բ���;
��5������ƽ�ⳣ������ʵ��ķ����ⶨ�����ģ����Ѿ����ij�¶��� NH3∙H2O��Һ�д������·�Ӧ��NH3∙H2O NH4++OH-
��֪0.10 mol��L-1
NH3∙H2O��Һ�У��ﵽƽ��ʱ��Cƽ����OH-��=4.2 �� 10-3mol��L-1��Cƽ����NH3∙H2O����C��ʼ��NH3∙H2O����ˮ�ĵ���ɺ��Բ���;
����pH��ֽ������Һ��pHֵ���������Cƽ����OH-�����ⶨ��ҺpHֵ�IJ�����______________��
�ڲ���Cƽ����NH3∙H2O���ķ��������_____________����������ƣ�
������¶��¸÷�Ӧ��ƽ�ⳣ��K.(д��������̣�����������2λ��Ч����)