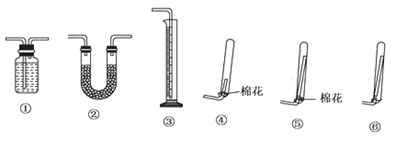
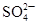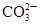��Ŀ����
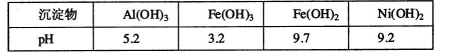
ij������Ni���ϴ�������Ҫ����Ni��������Al��Al203��Fe�������������ᡢ������ʡ�����������������������ʽ��ȫ����ʱ��Һ��pH���£�
���Ӻ����ϴ������Ƶ�NiSO4��7H2O���壬���������£�
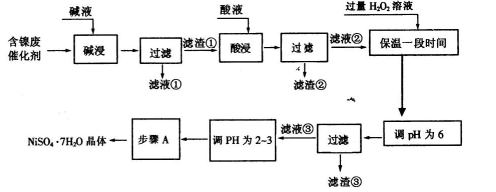
�����������Ϣ������ͼ���ش��������⣺
��1�����������Ŀ���dz�ȥ�����ϴ����е�___ _��
��2���������ʱ�����������___ _���������Һ���п��ܺ��еĽ���������___ ���������ӷ��ű�ʾ����
��3������pHΪ2��3��Ŀ����___ _��
��4������A�IJ���˳���Ǽ���Ũ������ȴ��____��____��
��5��NiSO4��ǿ����Һ����NaC1O���������Ƶü������ӵ�ص缫����NiOOH���÷�Ӧ�����ӷ���ʽΪ________��

���Ӻ����ϴ������Ƶ�NiSO4��7H2O���壬���������£�

�����������Ϣ������ͼ���ش��������⣺
��1�����������Ŀ���dz�ȥ�����ϴ����е�___ _��
��2���������ʱ�����������___ _���������Һ���п��ܺ��еĽ���������___ ���������ӷ��ű�ʾ����
��3������pHΪ2��3��Ŀ����___ _��
��4������A�IJ���˳���Ǽ���Ũ������ȴ��____��____��
��5��NiSO4��ǿ����Һ����NaC1O���������Ƶü������ӵ�ص缫����NiOOH���÷�Ӧ�����ӷ���ʽΪ________��
��13�֣���1��Al��Al2O3��2�֣� ��2��H2SO4��2�֣���Ni2����Fe2����2�֣�
��3����ֹ��Ũ���ᾧ������Ni2+ˮ�⣨2�֣� ��4���ᾧ�����ˣ�2�֣�
��5��2Ni2+��ClO-��4OH-��2NiOOH����Cl-��H2O��3�֣�
��3����ֹ��Ũ���ᾧ������Ni2+ˮ�⣨2�֣� ��4���ᾧ�����ˣ�2�֣�
��5��2Ni2+��ClO-��4OH-��2NiOOH����Cl-��H2O��3�֣�
�����������1�����������������Ϊ�˳�ȥ������������Al2O3�����������������Ժ�ǿ�Ӧ���ܽ�õ�ƫ�����Σ���Ӧ�����ӷ���ʽ�ֱ�Ϊ2Al+2OH-+2H2O��2AlO2-+3H2����Al2O3+2OH-��2AlO2-+3H2O��
��2���������ʱ��Ҫ���ܽ��������������ʼ�������������Ʊ�Ŀ���ǵõ�NiSO4?7H2O����˼������������µ����ʣ�������Ҫ������������������������ϡ���ᷴӦ����������������������Һ�������Һ���п��ܺ��еĽ���������Ni2+��Fe2+��
��3����������Һ��Ҫ����Ũ���ᾧ������Ϊ��ֹ������ˮ����������������������Ҫ������ҺpH��
��4������A�IJ���˳���Ǽ���Ũ������ȴ���ᾧ�����ˡ�
��5��NiSO4��ǿ����������NaClO���������Ƶü������ӵ�ص缫����NiOOH����˸÷�Ӧ�����ӷ���ʽ��2Ni2+��ClO-��4OH-��2NiOOH����Cl-��H2O��

��ϰ��ϵ�д�
 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
�����Ŀ