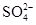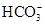��Ŀ����
�����£����и������ӻ������ָ����Һ���ܴ����������
| A�����ȳʺ�ɫ����Һ�У�MnO4����Al3����C2H5OH��SO42- |
| B��ˮ�������c(H��)��1��10��14 mol��L��1����Һ�У�Ca2����NH4����Cl����SiO32�� |
| C��c(H��)/c(OH��)��1012����Һ�У�NH4+��Al3����NO3-��ClO�� |
| D��c(NaHCO3)��0.1 mol��L��1����Һ�У�K����C6H5O����SO42-��CO32�� |
D
���������A��ʹ���ȳʺ�ɫ����ҺΪ������Һ����ʱMnO4�����C2H5OH����������ʴ������ԭ��Ӧ�����ܴ������档����B���������£�ˮ���������c(H��)= 1��10��7 mol/L.����ˮ�������c(H��)��1��10��14 mol/L<1��10��7 mol/L,˵����Һ�����Ի���ԡ�����Һ�����ԣ�������Ӧ��H++ SiO32��=H2SiO3��������Һ�ʼ��ԣ�������Ӧ��NH4��+OH-=NH3+H2O�����Զ����ܴ������档����C. c(H��)/c(OH��)��1012, c(H��)��c(OH��)��10-14.��ʽ������⣬�ɵ�c(H��)=0.1mol����ҺΪ���ԣ���ʱ������Ӧ��H++ ClO��=HClO.���ܴ������档D. NaHCO3��ѡ���е����Ӳ��ᷢ���κη�Ӧ�����Դ������档��ȷ��

��ϰ��ϵ�д�
�����Ŀ
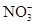 ��SCN��
��SCN��