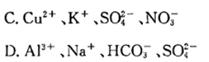��Ŀ����
ij������ˮ��Һ��ֻ���ܺ������������е������֣�Na+��NH4+��Ba2+��Cl-��CO32-��SO42-����ȡ����200 mL��Һ��������ʵ�飺�ٵ�һ�ݼ�����NaOH��Һ�����ȣ��ռ�������1.36 g;�ڵڶ��ݼ�����BaCl2��Һ�ø������12.54 g,����������ϴ�ӡ������������Ϊ4.66 g����������ʵ����ʵ�������Ʋ�����ȷ����( )
| A��һ��������Ba2+�����ܴ���NH4+ |
| B�������ܴ���Ba2+��Cl- |
| C��Na+һ�����ڣ���c(Na+)��0.2 mol/L |
| D��Na+��Cl-���ܴ��� |
C
����ɢ��п�֪���ռ�������1.36 gһ���ǰ�������n(NH4+)="0.08" mol������ɢ��п�֪������������ij���4.66 gΪ���ᱵ�����ʵ���Ϊ0.02 mol����һ�ֳ���7.88 gΪ̼�ᱵ�����ʵ���Ϊ0.04 mol��ԭ��Һn(CO32-)="0.04" mol,n(SO42-)="0.02" mol,������Ba2+����һ�����е�����NH4+��CO32-��SO42-��������ɵ����ʵ�����������ɵ����ʵ�������һ�����������ӣ������Ӳ�ȷ������û�������ӣ�n(Na+)="0.04" mol��������Cl-ʱ�����ۺ�����Cl-���ɵ���غ��֪n(Na+)������0.04 mol���������Ϊ200 mL����c(Na+)��0.2 mol/L��ֻ��ѡ��C��ȷ

��ϰ��ϵ�д�
 ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
�����Ŀ