��Ŀ����
����Ŀ��һ���¶��£���һ�ݻ�Ϊ5 L�ĺ����ܱ������г���0.4 mol SO2��0.2 mol O2��������Ӧ��2SO2(g)+O2(g)![]() 2SO3(g) ��H����196 kJ/mol������Ӧ�ﵽƽ��ʱ��������ѹǿ��Ϊ��ʼʱ��0.7������ش��������⣺
2SO3(g) ��H����196 kJ/mol������Ӧ�ﵽƽ��ʱ��������ѹǿ��Ϊ��ʼʱ��0.7������ش��������⣺
��1 ���жϸ÷�Ӧ�ﵽƽ��״̬�ı�־��___________(����ĸ)��
A��SO2��O2��SO3���ߵ�Ũ��֮��Ϊ2��1��2 B�������������ѹǿ����
C�������ڻ��������ܶȱ��ֲ��� D��SO3�����ʵ������ٱ仯
E��SO2���������ʺ�SO3�������������
��2����SO2��ת���ʣ�_______________���ڴﵽƽ��ʱ��Ӧ�ų�������Ϊ____________kJ���۴��¶��¸÷�Ӧ��ƽ�ⳣ��K��__________��
��3����ͼ��ʾƽ��ʱSO2�����������ѹǿ���¶ȱ仯�����ߣ���
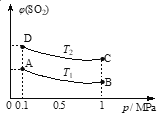
���¶ȹ�ϵ��T1________T2(���������������������ͬ)��
��ƽ�ⳣ����ϵ��KA_________KB��KA_________KD��
���𰸡� BDE 90% 35.28 20250 �� �� ��
��������(1)SO2��O2��SO3����Ũ�ȱ�Ϊ2��1��2,��ƽ��״̬�ޱ�Ȼ��ϵ,������Ϊ����ƽ��ı�־,����������ѹǿ�淴Ӧ���ж��仯,����ѹǿ����,����ƽ��;��������������,�����������,��������ܶ�Ҳ����,������Ϊƽ��״̬��־;SO3�����ʵ������淴Ӧ���ж������仯��,���䲻��ʱ,��ʾ��Ӧ�ѽ���ƽ��;SO2������������v(SO2)��,SO3����������v(SO3)��,��v(SO2)��=v(SO3)��,����v(SO2)��=v(SO2)��,����ƽ�⽨���ĸ�����־�����Կ���Ϊƽ��״̬��־����b��d��e��
(2) 2SO2(g)+O2(g)![]() 2SO3(g)
2SO3(g)
��ʼ(mol) 0.4 0.2 0
�仯(mol) x x/2 x
ƽ��(mol) 0.4-x 0.2-x/2 x
T��V�㶨,�����ѹǿ֮�ȵ������ʵ���֮��![]() =
=![]() ,���x=0.36,��SO2ת����Ϊ:
,���x=0.36,��SO2ת����Ϊ:![]() ��100%=90%���ų�����Ϊ:
��100%=90%���ų�����Ϊ:![]() kJ��mol-1��0.4 mol��90%="35.28" kJ,K=
kJ��mol-1��0.4 mol��90%="35.28" kJ,K=![]() =
=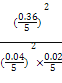 ="20" 250��
="20" 250��
(3)�÷�Ӧ�Ƿ��ȷ�Ӧ,�¶�����ƽ������,SO2�����������,����T2��T1,ƽ�ⳣ��ֻ���¶��й�,�¶Ȳ���,Kֵ����,��KA=KB,����,ƽ������,Kֵ��С,KA��KD��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ�����ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���á�
(1)��ͼ��N2(g)��H2(g)��Ӧ����1 mol NH3(g)�����������仯ʾ��ͼ����д��N2��H2��Ӧ���Ȼ�ѧ����ʽ��____________________________________________��

(2)��֪��ѧ���������γɻ��1 mol��ѧ���ų������յ���������λkJ��mol��1������֪�������ݣ�
��ѧ�� | HH |
|
����/kJ��mol��1 | 435 | 943 |
�Ը��ݱ��м�ͼ�����ݼ���N��H�ļ���________kJ��mol��1��
(3)��NH3����ԭNOx���������������������Ⱦ����֪��
4NH3(g)��3O2(g)===2N2(g)��6H2O(g) ��H1����a kJ��mol��1 ��
N2(g)��O2(g)===2NO(g) ��H2����b kJ��mol��1 ��
��1 mol NH3��ԭNO��N2����÷�Ӧ�����еķ�Ӧ�Ȧ�H3��________ kJ��mol��1(�ú�a��b��ʽ�ӱ�ʾ)��