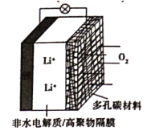
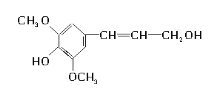
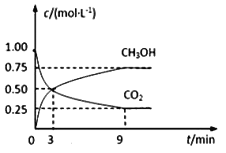
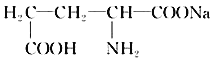��Ŀ����
����Ŀ����һ���¶������£��ס��������ݻ���ȵĺ����ܱ������о��������·�Ӧ��3A(g)+B(g)![]() xC(g)+D(s)�������ͨ��6molA��2molB��������ͨ��1.5molA��0.5molB��3molC��2molD����Ӧһ��ʱ��ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2��������������ȷ���ǣ� ��
xC(g)+D(s)�������ͨ��6molA��2molB��������ͨ��1.5molA��0.5molB��3molC��2molD����Ӧһ��ʱ��ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2��������������ȷ���ǣ� ��
A.ƽ��ʱ����A���������Ϊ0.4
B.ƽ��ʱ�ס�����������A��B�����ʵ���֮�Ȳ����
C.��ƽ��ʱ�������е�ѹǿ����ȣ�����������ѹǿ֮��Ϊ8��5
D.��ƽ��ʱ�ס�����������A�����ʵ�����ȣ���x=4
���𰸡�C
��������
A. C�����������Ϊ0.2����A��B��ռ80%����n(A)��n(B)=3��1������ƽ��ʱ����A���������Ϊ60%����A����
B. �ס�����������A��B��Ͷ�ϱȶ�����ϵ���ȣ�����ƽ��ʱ�ס�����������A��B�����ʵ���֮����ȣ���B����
C.��ƽ��ʱ�������е�ѹǿ����ȣ���ס���Ϊ�ȱȵ�Ч�ĵ�Чƽ�⣬x=4����Ͷ����6mol+2mol=8mol���ҵ�Ͷ����1.5mol+0.5mol+3mol=5mol������������ѹǿ֮��Ϊ8��5����C��ȷ��
D. ��ƽ��ʱ�ס�����������A�����ʵ�����ȣ���Ϊ������Ч�ĵ�Чƽ�⣬������ ����x=2����D����
����x=2����D����
ѡC��

 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�