��Ŀ����
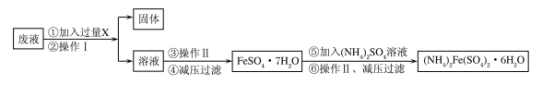
����Ŀ����֪X��YΪ��ѧ��ѧ�еij���Ԫ�أ�����ۺ������Ϊǿ�ᡣ������ͼ��ʾת����ϵ(��Ӧ���������ֲ�������ȥ)���ش��������⡣
![]()
(1)��A��B��C��D��Ϊ��X�Ļ������A��F�ķ����о�����10�����ӣ���
��F�Ļ�ѧʽΪ______��
�ڽ�1.92 gͭ����һ������D��Ũ��Һ��Ӧ����ͭ����ȫ��Ӧʱ�ռ�������1.12 L (��״��)����Ӧ���ĵ�D�����ʵ���Ϊ______mol��
(2)��A��B��C��D��Ϊ��Y�Ļ��������A������Ԫ����ɣ���A��Ħ������Ϊ34 g/mol����
�ٽ�ͭ����D��Ũ��Һ��Ӧ���õ���Һ�������ɣ��õ��İ�ɫ��������Ϊ______(�ѧʽ)��
�ڽ�Na2Y��Һ�μӵ�����������Һ�У��л�ɫ�������ɣ���д����������Ӧ�����ӷ���ʽ��______���ڸ÷�Ӧ������74.5 gNaClO����ԭ����ת�Ƶ��ӵ����ʵ���Ϊ______mol��
���𰸡�H2O 0.11 CuSO4 S2-+ClO-+H2O=S��+Cl-+2OH- 2
��������
��֪X��YΪ��ѧ��ѧ�еij���Ԫ�أ�X��Y����Ԫ������������ˮ�����Ϊǿ�ᡣ
(1)��A��B��C��D��Ϊ��XԪ�صĻ������A��F�ķ����о�����10�����ӣ���A��NH3��B��NO��C��NO2��D��HNO3��EΪO2��FΪH2O��
��������������֪��FΪH2O��
��Cu��Ũ���ᷴӦ����Cu(NO3)2��NO2��H2O������Cu����������Cu�����ʵ�����HNO3������Ϊ��������������ã����ݵ�Ԫ���غ���㷴Ӧ����HNO3�����ʵ�����
(2)��A��B��C��D��Ϊ��Y�Ļ��������A������Ԫ����ɣ���A��Ħ������Ϊ34 g/mol����YԪ��ΪSԪ�أ���A��H2S��B��SO2��C��SO3��D��H2SO4��E��O2��F��H2O��
��ͭ�����ᷴӦ��������ͭ������������ˮ�����õ���Һ������ͭ��Һ���������ɵõ��İ�ɫ����������CuSO4��
�ڻ�����Na2S�ʹ���������Һ��ǿ���Ի������ܷ�����Ӧ������Ϊ��ɫ��������Ȼ�����ˮ����ϻ��ϼ۵ı仯���
������������֪X��NԪ�أ�Y��SԪ�ء�
(1) A��NH3��B��NO��C��NO2��D��HNO3��EΪO2��FΪH2O��
��F��ˮ����ѧʽΪH2O��
��1.92 gCu�����ʵ���Ϊn(Cu)= ![]() =0.03 mol��Cu��Ũ���ᷴӦ����Cu(NO3)2��NO2��H2O������Ӧ���е�һ���̶ȣ���Ϊϡ���ᣬ��Ӧ����Cu(NO3)2��NO��H2O����Ӧ����������NO��NO2�����ʵ���n=1.12 L��22.4 L/mol=0.05 mol����Ӧ����Cu(NO3)2�����ʵ���Ϊ0.03 mol�����ݵ�Ԫ���غ㣬���ĵ���������ʵ���Ϊ0.03 mol��2+0.05 mol=0.11 mol��
=0.03 mol��Cu��Ũ���ᷴӦ����Cu(NO3)2��NO2��H2O������Ӧ���е�һ���̶ȣ���Ϊϡ���ᣬ��Ӧ����Cu(NO3)2��NO��H2O����Ӧ����������NO��NO2�����ʵ���n=1.12 L��22.4 L/mol=0.05 mol����Ӧ����Cu(NO3)2�����ʵ���Ϊ0.03 mol�����ݵ�Ԫ���غ㣬���ĵ���������ʵ���Ϊ0.03 mol��2+0.05 mol=0.11 mol��
(2)YԪ��ΪSԪ�أ�A��H2S��B��SO2��C��SO3��D��H2SO4��E��O2��F��H2O��
��ͭ��Ũ���ᷴӦ����CuSO4��SO2��H2O�����õ���Һ������ͭ��Һ���������ɵõ���������CuSO4��
�ڻ�����Na2S���л�ԭ�ԣ���NaClO���������ԣ�������ǿ���Ի������ܷ�����Ӧ��������ɫ��������ó���ΪS��S2-����������S��ClO-����ԭ����Cl-��ͬʱ��ˮ���ɣ��÷�Ӧ�����ӷ�Ӧ����ʽ��S2-+ClO-+H2O=S��+Cl-+2OH-���ڷ�Ӧ��ÿ��1 mol NaClO��Ӧ��ת��2 mol���ӣ�74.5 g NaClO�����ʵ���n(NaClO)= ![]() =1 mol����Ӧת�Ƶ��ӵ����ʵ���Ϊ2 mol��
=1 mol����Ӧת�Ƶ��ӵ����ʵ���Ϊ2 mol��

����Ŀ�����ϴ�ѧ֣�Žܵ�3λ��ʦ������Ժ����ˮ������ԭ���Ʊ�As2O3�����нϸߵ�ʵ��Ӧ�ü�ֵ��ij���������ˮ����H3AsO3��H2SO4��Fe2(SO4)3��Bi2(SO4)3�ȣ����ø÷�ˮ��ȡAs2O3��������ͼ��ʾ��

�����������ӳ���pHֵ����(20��)
10-1 | 10-2 | 10-3 | 10-4 | 10-5 | |
Fe3+ | 1.8 | 2.2 | 2.5 | 2.9 | 3.2 |
Cu2+ | 4.7 | 5.2 | 5.7 | 6.2 | 6.7 |
(1)Ϊ�˼ӿ��к��̵����ʣ����Բ�ȡ�Ĵ�ʩ��______________(д��һ�������Ĵ�ʩ����)��
(2)�����еijɷ֣�����Bi(OH)3�����⣬����_________��
(3)A����ѭ�����ã�A�Ļ�ѧʽΪ_________������Һ1�У�����NaOH����pHΪ8��Ŀ����_______��
(4)Cu3(AsO3)2��������һ������ˮ���ɽ��ϣ�ͨ��SO2���ù��̵Ļ�ѧ��Ӧ����ʽ��__________��
(5)����һ����Һ�̱ȣ���ˮ����Cu3(AsO3)2�����У����ɽ��ϡ�����Ӧ�¶�Ϊ25�棬SO2����Ϊ16L/h��Һ�̱ȡ�ʱ����顢ͭ�����ʵ�Ӱ����ͼ�ס�����ʾ����ѡ�������˵�Һ�̱ȡ���Ӧʱ�䣺______________��_______________��

(6)һ�������£����ۻ�(As4S4)�Ʊ�As2O3��ת����ϵ��ͼ��ʾ������Ӧ�У�1mol As4S4(����AsԪ�صĻ��ϼ�Ϊ+2��)�μӷ�Ӧʱ��ת��28mole-��������aΪ_______(�ѧʽ)��