��Ŀ����
ij�о���ѧϰС������Ʒ������ʽ���������ƣ�
�������Ǹ�С��ͬѧ���ʵ�����Ʊ������ļ��ַ���
����Ϊ���е��� ������ĸ,��ѡ�����֣���
�÷�Ӧ�Ļ�ѧ����ʽ
�Ƹ�С��ͬѧ��1 mol O2��NH3�Ļ�����嵼���ܱ������У��ڴ����������¼��ȣ�ʹ֮��ַ�Ӧ���ָ������³�ѹ�¡������������еķ�Ӧ�����������������Ӷ���������������Ӧ��
4NH3 + 5O2 4NO + 6H20��4NO + 3O2 + 2H2O = 4HNO3��ԭ�����������x mol O2���������������y mol HNO3������������ע��һ������H2O���õ���Һ��
4NO + 6H20��4NO + 3O2 + 2H2O = 4HNO3��ԭ�����������x mol O2���������������y mol HNO3������������ע��һ������H2O���õ���Һ��
������Һ�е����̪����Һ�ʺ�ɫ��y = ����x�Ĵ���ʽ��ʾ����ͬ��
x��ȡֵ��Χ ��
���������в�����ˮ�����壬�������ڿ����б�Ϊ����ɫ������Һ�е���ʯ���Һ�ʺ�ɫ��y = ��x��ȡֵ��Χ
�������Ǹ�С��ͬѧ���ʵ�����Ʊ������ļ��ַ���
| A������粒�����������ƹ����ϼ��� | B�������Ȼ�粒��� |
| C���Ȼ�粒�����������ƹ����ϼ��� | D����Ũ�������Ũ��ˮ�� |
�÷�Ӧ�Ļ�ѧ����ʽ
�Ƹ�С��ͬѧ��1 mol O2��NH3�Ļ�����嵼���ܱ������У��ڴ����������¼��ȣ�ʹ֮��ַ�Ӧ���ָ������³�ѹ�¡������������еķ�Ӧ�����������������Ӷ���������������Ӧ��
4NH3 + 5O2
 4NO + 6H20��4NO + 3O2 + 2H2O = 4HNO3��ԭ�����������x mol O2���������������y mol HNO3������������ע��һ������H2O���õ���Һ��
4NO + 6H20��4NO + 3O2 + 2H2O = 4HNO3��ԭ�����������x mol O2���������������y mol HNO3������������ע��һ������H2O���õ���Һ��������Һ�е����̪����Һ�ʺ�ɫ��y = ����x�Ĵ���ʽ��ʾ����ͬ��
x��ȡֵ��Χ ��
���������в�����ˮ�����壬�������ڿ����б�Ϊ����ɫ������Һ�е���ʯ���Һ�ʺ�ɫ��y = ��x��ȡֵ��Χ
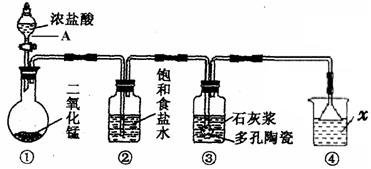
��C 2NH4Cl + Ca(OH)2=aCl2 + 2NH3�� + 2H2O
�Ƣ�O��x��5/9 y="O "
��5/9��x��2/3 y=9x-5/3
�Ƣ�O��x��5/9 y="O "
��5/9��x��2/3 y=9x-5/3
��

��ϰ��ϵ�д�
 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�
�����Ŀ
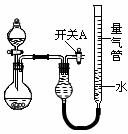
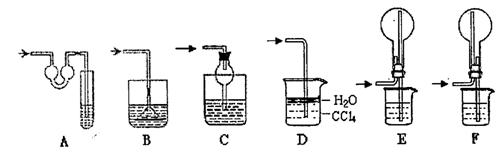
 3��ʵ�����ʣ���NH3�����մ��������¸���β������װ���У��ʺ�������NH3�������ܷ�ֹ��������
3��ʵ�����ʣ���NH3�����մ��������¸���β������װ���У��ʺ�������NH3�������ܷ�ֹ��������