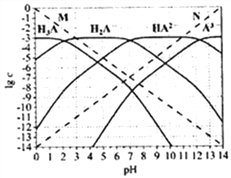
��Ŀ����
����Ŀ������«�����ṹ��ʽ�� ����������ϩ���ӻ�������п�������������Ԥ����Ѫ�ܼ��������á�ij��������������ºϳ�·�ߣ�
����������ϩ���ӻ�������п�������������Ԥ����Ѫ�ܼ��������á�ij��������������ºϳ�·�ߣ�

��֪��

����������Ϣ�ش��������⣺
��1������«���ķ���ʽ��___________��
��2��C��D�ķ�Ӧ������_______��E��F�ķ�Ӧ������_________��
��3��������A����FeCl3��Һ������ɫ��Ӧ������NaHCO3��Ӧ�ų�CO2���Ʋ���1H�˴Ź����ף�H-NMR������ʾ��ͬ��ѧ��������ԭ�Ӹ�����Ϊ________��
��4��д��A��B��Ӧ�Ļ�ѧ����ʽ��___________________________________��
��5��д���ṹ��ʽ��D______________��E_______________��
��6��������![]() ������������������ͬ���칹�干_________�֣�
������������������ͬ���칹�干_________�֣�
���ܷ���������Ӧ���ں������ұ�����ֻ�����ֲ�ͬ��ѧ��������ԭ�ӡ�
д�����в���Ӧ��ͬ���칹��Ľṹ��ʽ��_____________________��
���𰸡� C14H12O3 ȡ�� ��ȥ 1:1:2:6 ![]() +CH3OH
+CH3OH![]() +H2O
+H2O 
 3
3 ![]()
��������F�����廯��Ӧ���ɰ�«��������F�ķ���ʽ��������Ϣ֪��F�к���3�����ǻ���F�Ľṹ��ʽΪ![]() ��E������ȥ��Ӧ����F������F�Ľṹ��ʽ֪E�Ľṹ��ʽΪ
��E������ȥ��Ӧ����F������F�Ľṹ��ʽ֪E�Ľṹ��ʽΪ ��D�����ӳɷ�Ӧ����E������E�Ľṹ��ʽ֪D�Ľṹ��ʽΪ
��D�����ӳɷ�Ӧ����E������E�Ľṹ��ʽ֪D�Ľṹ��ʽΪ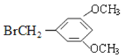 ��C����ȡ����Ӧ����D�����C�ķ���ʽ֪C�Ľṹ��ʽΪ
��C����ȡ����Ӧ����D�����C�ķ���ʽ֪C�Ľṹ��ʽΪ![]() ��B��Ӧ����C����������Ϣ��B�ķ���ʽ֪��B�Ľṹ��ʽΪ��
��B��Ӧ����C����������Ϣ��B�ķ���ʽ֪��B�Ľṹ��ʽΪ��![]() ��A����������Ӧ����B������A�ķ���ʽ��B�Ľṹ��ʽ֪��A�Ľṹ��ʽΪ
��A����������Ӧ����B������A�ķ���ʽ��B�Ľṹ��ʽ֪��A�Ľṹ��ʽΪ![]() ��
��
��1�����ݰ���«���Ľṹ��ʽ֪�������ʽΪC14H12O3����2��C��D�ķ�Ӧ������ȡ����Ӧ��E��F�ķ�Ӧ��������ȥ��Ӧ����3������ȷ���Ļ�����A�Ľṹ��ʽ��![]() ����֪������1H�˴Ź����ף�H-NMR������ʾ��4�ֲ�ͬ��ѧ��������ԭ�ӣ��������Ϊ1��1��2��6����4��AΪ
����֪������1H�˴Ź����ף�H-NMR������ʾ��4�ֲ�ͬ��ѧ��������ԭ�ӣ��������Ϊ1��1��2��6����4��AΪ![]() ����״�����������Ӧ����B��BΪ
����״�����������Ӧ����B��BΪ![]() ��A��B��Ӧ�Ļ�ѧ����ʽΪ��
��A��B��Ӧ�Ļ�ѧ����ʽΪ��![]() +CH3OH
+CH3OH![]() +H2O ����5�������Ϸ�����֪��D�Ľṹ��ʽΪ��
+H2O ����5�������Ϸ�����֪��D�Ľṹ��ʽΪ��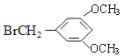 ��E�Ľṹ��ʽΪ��
��E�Ľṹ��ʽΪ�� ����6�����ݸ����������ܷ���������Ӧ����ȷ�����ӽṹ����ȩ�����ں������ұ�����ֻ�����ֲ�ͬ��ѧ��������ԭ�ӣ���ȷ���������ж���ȡ�����������ڶ�λ������
����6�����ݸ����������ܷ���������Ӧ����ȷ�����ӽṹ����ȩ�����ں������ұ�����ֻ�����ֲ�ͬ��ѧ��������ԭ�ӣ���ȷ���������ж���ȡ�����������ڶ�λ������![]() ��ͬ���칹����3�֣�
��ͬ���칹����3�֣�![]() ��
��![]() ��
��![]() �����в���Ӧ��ͬ���칹��Ľṹ��ʽΪ
�����в���Ӧ��ͬ���칹��Ľṹ��ʽΪ![]() ��
��

����Ŀ�����и��鷴Ӧ���������ʾ�Ϊ��Ӧ�����Ӧ�տ�ʼʱ���ų�H2�������������� ��
A | Mg 0.1mol | 6mol�� L��1����10mL | 60�� |
B | Mg 0.1mol | 3mol��L��1����10mL | 60�� |
C | Fe 0.1mol | 3mol��L��1����10mL | 60�� |
D | Mg 0.1mol | 3mol��L��1����10mL | 60�� |
A. A B. B C. C D. D