��Ŀ����
��4�֣���0.1 g MnO2��ĩ����50 mL H2O2��Һ�У��ڱ�״��
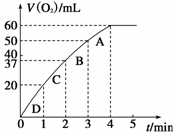 �·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ�����ͷ�Ӧ���ʱ仯��
�·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ�����ͷ�Ӧ���ʱ仯��
ԭ�� ������H2O2�ij�
ʼ���ʵ���Ũ��Ϊ ��(������λ��Ч����)
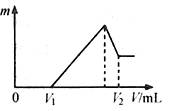
��8�֣�һ���¶��£���3 molA�����1mol B����ͨ��һ�ݻ��̶�Ϊ2L���ܱ������У��������·�Ӧ��3A(g)��B(g) ![]() xC(g)������д���пհף�
xC(g)������д���пհף�
��1����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L����1min�ڣ�B��ƽ����Ӧ����Ϊ ��XΪ ��
��2������Ӧ��2min�ﵽƽ�⣬ƽ��ʱC��Ũ�� 0.8mol/L������ڣ�С�ڻ���ڡ�����
��3������֪��ƽ��ʱ���������ڻ��������ѹǿΪp�����������ʼѹǿΪp0��������p0��p����ʾ��ƽ��ʱ��Ӧ��A��ת���ʧ�(A)Ϊ ��
��4���ܹ�˵���÷�Ӧ�ﵽƽ��ı�־�ǣ� ��
A.�����ڻ��������ܶȱ��ֲ��� B.v(A)=3v(B) C.A��B��Ũ��֮��Ϊ3:1
D.��λʱ��������3n molA��ͬʱ����n molB E.��������ƽ����Է�������
�����ŷ�Ӧ�Ľ��У�H2O2 ��Ũ�ȼ�С����Ӧ���ʼ�����2�֣��� 0.11mol/L(2��)
��(1)��0.2mol/(L.min) ��2�֣����� 2��1�֣�
(2)������2�֣��� (3)��������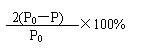 ������������ ��2�֣������� (4)��DE��1�֣�������
������������ ��2�֣������� (4)��DE��1�֣�������