��Ŀ����
��15�֣�������Ԫ��X��Y��Z��Ԫ�����ڱ��е�λ������ͼ��ʾ�����ǵ�������֮��Ϊ21��
��1����Ԫ����Z�γ�ԭ�Ӹ�����Ϊ1��1�Ļ���������ʽΪ_____________���û���������д���__________������ۼ��������Ӽ�������
��2��Y����������ˮ������Y���⻯��ǡ����ȫ��Ӧʱ���������ˮ��Һ�����ԣ���ԭ����_________________________________________���������ӷ���ʽ��ʾ��
����Һ�и�������Ũ���ɴ�С˳��Ϊ______________________________��
��3���ٺ����£����ݻ�Ϊ2L�ĸ��������г���2mol H2��2mol Y�ĵ��ʣ�5���Ӻ�Ӧ�ﵽƽ��ʱ����ʱY�ĵ���Ϊ1��8mol�������ķ�Ӧ����Ϊ
ƽ��ʱ������ѹǿ�뷴Ӧǰѹǿ��Ϊ_______ ___��
�����÷�Ӧ�ں��º�ѹ�����½��У�����������ͬ������Ӧ�ﵽƽ��ʱ��H2��ת���ʱ�����������H2��ת����_______�������С������ͬ������
��4�������£���X�������̬�⻯��3��2g����������ȫȼ�պ�ָ������£��ų�a kJ ����������д���÷�Ӧ���Ȼ�ѧ����ʽ��____________________________________��
��1�� ��1�֣� ���ۼ� ��1�֣�
��2�� NH4+ + H2ONH3��H2O+ H+ ��2�֣�
C��NO3-����C��NH4+����C��H+����C��OH-�� ��2�֣�
��3��0��04mol/��L��min���� 9:10 �� ����2�֣���6�֣�
��4��CH4��g�� + 2O2��g�� = CO2��g�� + 2H2O��l�� ��H= -5a KJ/mol ��3�֣�
����:

 ������ȫ��������ϵ�д�
������ȫ��������ϵ�д���2013?����ģ�⣩������Ԫ��X��Y��Z��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������Y������������������������ȣ�����˵����ȷ���ǣ�������
|
| Ԫ�� | �۵㣨�棩 | �е㣨�棩 | ��ˮ���� | ��Һ��ĵ����� |
| X | -183 | -162 | ����ˮ��ӦҲ������ˮ | ������ |
| Y | -102 | 19 | ���ȣ��γɵ��������� | ������ |
| Z | 680 | - | ���ҷ�Ӧ����H2����Һ�ʼ��� | ���� |
| A��X��Y��Z |
| B��Z��X��Y |
| C��Y��X��Z |
| D��Z��Y��X |
| X | Y | ||||
| Z | M | R | |||
| A��Ԫ��X��Y�����γ��������ϵ���̬������ |
| B��ԭ�Ӱ뾶�Ĵ�С˳��Ϊ��r��Z����r��M����r��R�� |
| C��Ԫ�ص�����������Ӧˮ���������Rǿ��M |
| D������������Ԫ��R��Z�γɵĻ������ˮ��Һ���Եõ��û�����ľ��� |
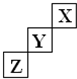 ������Ԫ��X��Y��Z��Ԫ�����ڱ��е�λ����ͼ��ʾ������˵����ȷ���ǣ�������
������Ԫ��X��Y��Z��Ԫ�����ڱ��е�λ����ͼ��ʾ������˵����ȷ���ǣ�������