��Ŀ����
����Ŀ��(1)�������ӿɿ�������CH3����CH2����![]() ��
��![]() �Ƚ�϶��ɵġ���д��ͬʱ�������������������Ľṹ��ʽ��___________ ������������ϩ���ӳɵõ������ϩ��������___________�֡�
�Ƚ�϶��ɵġ���д��ͬʱ�������������������Ľṹ��ʽ��___________ ������������ϩ���ӳɵõ������ϩ��������___________�֡�
�� ������ͬʱ��������4�ֻ��ţ��� ����������̼ԭ�������٣�
�� ��������һ�ȴ���ͬ���칹�����Ŀ���١�
(2)̼ԭ����Ϊ8�ĵ�ϩ���У���HBr�ӳɲ���ֻ��һ�ֽṹ�����������ĵ�ϩ����_______�֡�
(3)��֪ϩ��ͨ��������������п��ˮ�����õ�ȩ��ͪ�����磺
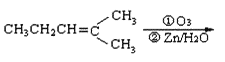 CH3CH2CHO+
CH3CH2CHO+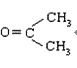
������Ӧ�������ƶ���������̼̼˫����λ�á�
ij��A�ķ���ʽΪC6H10����������ת������![]() ������A�Ľṹ�ɱ�ʾΪ_______________��
������A�Ľṹ�ɱ�ʾΪ_______________��
(4) ��д��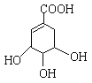 �����������Ľ����Ʒ�����Ӧ�Ļ�ѧ����ʽ_________��
�����������Ľ����Ʒ�����Ӧ�Ļ�ѧ����ʽ_________��
���𰸡�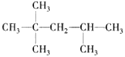 2 3
2 3 ![]()
 +4Na��
+4Na��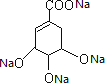 +2H2��
+2H2��
��������
(1)���ij����������ͬʱ������4�ֻ��ţ�����-CH2-��![]() ��
��![]() ������һ����ʣ�µ�Ϊ������������ͨʽȷ��̼ԭ�Ӹ�����ȷ���ṹ��ʽ��Ȼ�����һ�ȴ���ͬ���칹�����Ŀ���ٷ������
������һ����ʣ�µ�Ϊ������������ͨʽȷ��̼ԭ�Ӹ�����ȷ���ṹ��ʽ��Ȼ�����һ�ȴ���ͬ���칹�����Ŀ���ٷ������
(2)̼ԭ����Ϊ8�ĵ�ϩ���У���HBr�ӳɲ���ֻ��һ�ֽṹ��˵�������ʾ��жԳƽṹ���ݴ˷����жϿ��ܵĽṹ��
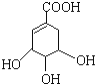
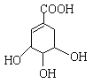
(3)ij��A�ķ���ʽΪC6H10��A�в����Ͷ�=![]() =2����A��������̼̼˫����һ��̼̼��������ڻ�״�ṹ�Һ���һ��̼̼˫��������Aͨ��������������п��ˮ�����õ�
=2����A��������̼̼˫����һ��̼̼��������ڻ�״�ṹ�Һ���һ��̼̼˫��������Aͨ��������������п��ˮ�����õ�![]() �������Ϣ������дA�Ľṹ��ʽ��
�������Ϣ������дA�Ľṹ��ʽ��
(4)�ǻ����Ȼ������Ʒ�Ӧ�����������ݴ���д��Ӧ�ķ���ʽ��
(1)���ij����������ͬʱ������4�ֻ��ţ�����-CH2-��![]() ��
��![]() ������һ����ʣ�µ�Ϊ��������ĸ�����x����������ͨʽ֪2(x+3)+2=2+1+3x��x=5����������Ӧ���е�̼ԭ������8���������Ľṹ��ʽ��3�֣��ֱ�Ϊ��
������һ����ʣ�µ�Ϊ��������ĸ�����x����������ͨʽ֪2(x+3)+2=2+1+3x��x=5����������Ӧ���е�̼ԭ������8���������Ľṹ��ʽ��3�֣��ֱ�Ϊ��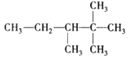 ��
��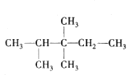 ��
��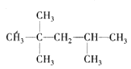 ����һ�ȴ��������ֱ�Ϊ��5�֡�5�֡�4�֣�һ�ȴ���ͬ���칹�����Ŀ���ٵ�Ϊ
����һ�ȴ��������ֱ�Ϊ��5�֡�5�֡�4�֣�һ�ȴ���ͬ���칹�����Ŀ���ٵ�Ϊ ��
�� ����ϩ���ӳɵõ���̼̼˫��������2��λ��(
����ϩ���ӳɵõ���̼̼˫��������2��λ��(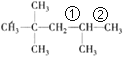 )������ϩ��������2�֣��ʴ�Ϊ��
)������ϩ��������2�֣��ʴ�Ϊ�� ��2��
��2��
(2)̼ԭ����Ϊ8�ĵ�ϩ���У���HBr�ӳɲ���ֻ��һ�ֽṹ��˵�������ʾ��жԳƽṹ�����ܵ�̼�ܽṹ��![]() ��
��![]() ��
��![]() ��3�֣��ʴ�Ϊ��3��
��3�֣��ʴ�Ϊ��3��
(3)ij��A�ķ���ʽΪC6H10��A�IJ����Ͷ�=![]() =2
=2![]() ��˵��A�к���һ�����Һ���һ��̼̼˫����������ȩ��ת��Ϊ̼̼˫������A�ṹ��ʽ����AΪ
��˵��A�к���һ�����Һ���һ��̼̼˫����������ȩ��ת��Ϊ̼̼˫������A�ṹ��ʽ����AΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]()
(4) �к����ǻ����Ȼ���̼̼˫���������ǻ����Ȼ������Ʒ�Ӧ������������Ӧ�ķ���ʽΪ
�к����ǻ����Ȼ���̼̼˫���������ǻ����Ȼ������Ʒ�Ӧ������������Ӧ�ķ���ʽΪ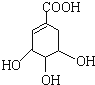 +4Na��
+4Na�� +2H2�����ʴ�Ϊ��
+2H2�����ʴ�Ϊ��  +4Na��
+4Na�� +2H2����
+2H2����

����Ŀ������ʱ�����Թ���ͨ������X����ͼ��������ʵ����Ԥ���������ʵ�����������
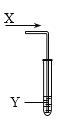
ѡ�� | ����X | �Թ��е�����Y | Ԥ������ |
A | ��ϩ | Br2��CCl4��Һ | ��Һ��ɫ����ȥ������Һ���Ϊ�������� |
B | SO2 | Ʒ����Һ | ��Һ��ɫ����ȥ |
C | ���� | ����KMnO4��Һ | ������������Һ��ɫ���� |
D | ���� | FeCl2��Һ��dz��ɫ�� | ��Һ��dz��ɫ��Ϊ��ɫ |
A. AB. BC. CD. D