��Ŀ����
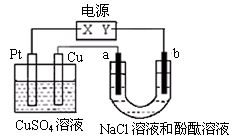
��15�֣����ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺

��1����X��Y���Ƕ��Ե缫��a��NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����
�� Y�缫�ϵĵ缫��ӦʽΪ ��
��X�������۲쵽�������� ��
�ڵ���ܷ�Ӧ���ӷ���ʽΪ ��
��2��Ҫ�������ı����ͭ��ֹ������ʴ����
�� Y�缫�IJ����� ��ѡ�������ͭ��п�����缫��Ӧʽ�� �����Һaѡ�� ��Һ������������Ũ�� ��ѡ������С�䣩��
�������ǰX��Y���缫��������ͬ�������ɺ�����ȡ��ϴ������ɡ�����������������Ϊ5.12 g������ʱ��·��ͨ���ĵ���Ϊ mol��
�۶Ʋ������ͭ���ȶ�п�������ױ���ʴ�����Ҫ˵��ԭ��:
��

��1����X��Y���Ƕ��Ե缫��a��NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����
�� Y�缫�ϵĵ缫��ӦʽΪ ��
��X�������۲쵽�������� ��
�ڵ���ܷ�Ӧ���ӷ���ʽΪ ��
��2��Ҫ�������ı����ͭ��ֹ������ʴ����
�� Y�缫�IJ����� ��ѡ�������ͭ��п�����缫��Ӧʽ�� �����Һaѡ�� ��Һ������������Ũ�� ��ѡ������С�䣩��
�������ǰX��Y���缫��������ͬ�������ɺ�����ȡ��ϴ������ɡ�����������������Ϊ5.12 g������ʱ��·��ͨ���ĵ���Ϊ mol��
�۶Ʋ������ͭ���ȶ�п�������ױ���ʴ�����Ҫ˵��ԭ��:
��
��1����2Cl--2e-=Cl2����2�֣� �����ݲ�������Һ��죨2�֣�
��2Cl- +2H2O���2OH-+Cl2��+H2����2�֣�
��2���ٴ�ͭ��1�֣�Cu-2e-=Cu2+��2�֣� CuSO4(����������Ҳ����) ��1�֣����䣨1�֣��� 0.08mol��2�֣�
������ͭ���ã��Ʋ��ƻ����ڳ�ʪ�Ļ������γ�ԭ��أ��������ĸ�ʴ(����������Ҳ����) ��2�֣�
��2Cl- +2H2O���2OH-+Cl2��+H2����2�֣�
��2���ٴ�ͭ��1�֣�Cu-2e-=Cu2+��2�֣� CuSO4(����������Ҳ����) ��1�֣����䣨1�֣��� 0.08mol��2�֣�
������ͭ���ã��Ʋ��ƻ����ڳ�ʪ�Ļ������γ�ԭ��أ��������ĸ�ʴ(����������Ҳ����) ��2�֣�
��1����ͼ���ã����Ӵ���ӵ�Դ�ĸ���������ص�����X����Һ�е������ӣ�Cl����OH-��������X��������Y��������Y����ʧ���ӵķ�Ӧ������������ӵ�Դ������������Һ�е������ӣ�Na+��H+��������Y��������X��
�ٹ�������ӦΪ��Y��2Cl--2e-=Cl2�����ŵ�������Cl����OH-����X��2H++2e-= H2�����ŵ�������H+��Na+����Ϊ������H+����ˮ�ĵ���ƽ�⣨H2O H++ OH-�������ƶ���ʹC��OH-������C��OH-����C��H+��������Һ��죻
H++ OH-�������ƶ���ʹC��OH-������C��OH-����C��H+��������Һ��죻
���ܻ�ѧ����ʽΪ2 NaCl+2H2O���2NaOH+Cl2��+H2��
��2��Ҫ�������ı����ͭ�������������������ô�ͭ����������������ӦΪ:
��������Cu-2e-= Cu2+��Cu2++2e-= Cu���ʵ���������Һ��C��Cu2+�����䣻
������ǰX��Y���缫��������Ϊm g�����ݵ缫��Ӧʽ�жϣ������������������a g��������������a g������(m+a)-(m-a)=" 5.12" g����a="2.56" g��n��Cu��="2.56" g/64g.mol-1="0.04" mol��Cu����2e-����n��e-��="2" n��Cu��=0.08mol��
�۶Ʋ������ͭ���У�����ͭ���ã��ʼ������ĸ�ʴ��
�Ʋ������п���У�п�������ã��ʼ���п�ĸ�ʴ��
�ٹ�������ӦΪ��Y��2Cl--2e-=Cl2�����ŵ�������Cl����OH-����X��2H++2e-= H2�����ŵ�������H+��Na+����Ϊ������H+����ˮ�ĵ���ƽ�⣨H2O
 H++ OH-�������ƶ���ʹC��OH-������C��OH-����C��H+��������Һ��죻
H++ OH-�������ƶ���ʹC��OH-������C��OH-����C��H+��������Һ��죻���ܻ�ѧ����ʽΪ2 NaCl+2H2O���2NaOH+Cl2��+H2��
��2��Ҫ�������ı����ͭ�������������������ô�ͭ����������������ӦΪ:
��������Cu-2e-= Cu2+��Cu2++2e-= Cu���ʵ���������Һ��C��Cu2+�����䣻
������ǰX��Y���缫��������Ϊm g�����ݵ缫��Ӧʽ�жϣ������������������a g��������������a g������(m+a)-(m-a)=" 5.12" g����a="2.56" g��n��Cu��="2.56" g/64g.mol-1="0.04" mol��Cu����2e-����n��e-��="2" n��Cu��=0.08mol��
�۶Ʋ������ͭ���У�����ͭ���ã��ʼ������ĸ�ʴ��
�Ʋ������п���У�п�������ã��ʼ���п�ĸ�ʴ��

��ϰ��ϵ�д�
 ���������ν�ϵ�д�
���������ν�ϵ�д�
�����Ŀ