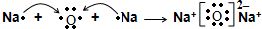��Ŀ����
17�����������ĺϳ�·����ͼ��ʾ��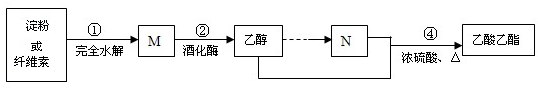
�ش��������⣺
��1�������˶�Ҫ����M�ĺ�����ʵ������������������ͭ�������Լ�����M��
��2����ҵ�ϳ��÷�ӦA $\stackrel{H_{2}O}{��}$ �Ҵ���A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ��A�Ľṹ��ʽCH3CH2OH���÷�Ӧ���л���Ӧ����Ϊ�ӳɷ�Ӧ��
��3��N��ϡ��Һ�����������г��õĵ�ζƷ��N�������ɼ���-CH3�����Ȼ�����ԭ�������ƣ����ɵģ�
���� ���۷���ˮ�ⷴӦ���������ǣ��������ھƻ�ø������ת��Ϊ�Ҵ����Ҵ���������������N�����Ҵ���N��Ӧ����������������NΪCH3COOH��
��1��ʵ���Ҽ��������ǣ�������������ͭ�������Լ��������Ǻ���ȩ��������������ͭ����ש��ɫ�������������Լ�����������
��2��A�IJ���ͨ����������һ������ʯ�ͻ���ˮƽ��A����ϩ����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ���
��3��NΪ���ᣬ��������ɼ����Ȼ����ɣ�
��� �⣺��1��������ϡ����������ˮ�����������ǣ�ˮ��ķ���ʽΪ��C6H10O5��n+nH2O$\stackrel{ϡ����}{��}$nC6H12O6�������Ǻ�����������ͭ�ڼ��������·�����Ӧ�������������ש��ɫ������ͭ����Ӧ�ķ���ʽΪCH2OH��CHOH��4CHO+2Cu��OH��2$\stackrel{��}{��}$CH2OH��CHOH��4COOH+Cu2O+2H2O����������ˮԡ���������·���������Ӧ����Ӧ�ķ���ʽΪCH2OH��CHOH��4CHO+2[Ag��NH3��2]OH$\stackrel{ˮԡ}{��}$CH2OH��CHOH��4COONH4+2Ag��+3NH3+H2O������������
�ʴ�Ϊ������������ͭ�������Լ���
��2��A�IJ���ͨ����������һ������ʯ�ͻ���ˮƽ��A����ϩ����ϩ����̼̼˫������ˮ�����ӳɷ�Ӧ��CH2=CH2+H2O$��_{��}^{����}$CH3CH2OH��
�ʴ�Ϊ��CH2=CH2+H2O$��_{��}^{����}$CH3CH2OH��
��3������dz��õĵ�ζƷ������Ҫ�ɷ�Ϊ���ᣬ����ṹ��ʽΪ��CH3COOH����������ɼ����Ȼ����ɣ�
�ʴ�Ϊ���Ȼ���
���� ���⿼���л����ƶϣ��漰�ǡ�ϩ������������������ת������Ŀ�Ѷ��еȣ����ضԻ���֪ʶ�Ĺ��̣�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | X��Y��Z��W��ԭ�Ӱ뾶���μ�С | |
| B�� | W��X�γɵĻ�������ֻ���ܺ������Ӽ� | |
| C�� | �ڹ�ҵ��X��Y������ֱ���ü��ȷֽ�ķ���ұ�� | |
| D�� | ��W��Y��ԭ���������5��������γɵĻ�����Ļ�ѧʽһ��ΪY2W3 |
| A�� | ͨ��������ˮ�� | B�� | �ڿ�����ȼ�� | ||
| C�� | ͨ�뱥��ʳ��ˮ�� | D�� | ͨ�����ʯ��ˮ |
| A�� | ����������������Ӧ | B�� | ����������ˮ��Ӧ | ||
| C�� | ϡ������þ��Ӧ | D�� | Ũ������̼��Ӧ |
| A�� | FeS+HCl | B�� | K2S+HCl | C�� | Na2S+CH3COOH | D�� | K2S+H2SO3 |
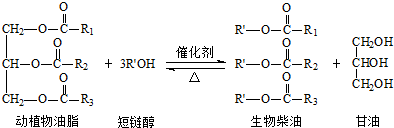
��������������ǣ�������
| A�� | ��������Dz�ͬ����ɵĻ���� | B�� | ��ֲ����֬�Ǹ߷��ӻ����� | ||
| C�� | ���ع��͡��������Ʊ�������� | D�� | ��������ɿ�������Դ�Ƶ� |
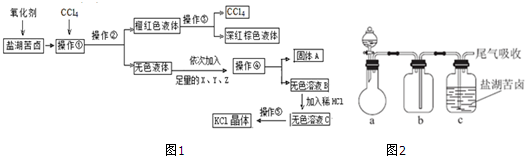

 C
C ��
�� ���÷�Ӧ����Ϊ��ȥ��Ӧ��
���÷�Ӧ����Ϊ��ȥ��Ӧ�� ��
��