��Ŀ����
12��ͬѧ�Ƕ���������ɣ�����ӳ�������ȡ������ʱ��������������һ����ƿ�𣿹رպ���ʱ��������β��������һ����ʶ�����������ѧ��֪ʶ���ش��������⣺��1�����Ͻ�ͷ���Ƿ��ֲ��ٹ������ͳ����ӡ�С�CNG����־����������������Ȼ����Ϊȼ�ϵ������� �ƹ���һ�ĸ����ҪĿ����C
A���ӳ������������� B���ٽ��ط����÷�չ
C�����ٴ�����Ⱦ D����Լ��
��2�����������Ľṹ�����������������Ľṹ���ƣ���д�������ƣ�
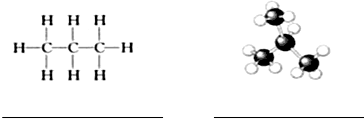
��3������ͱ���Ļ��������ȫȼ�պ��Ƚ�����ͨ��Ũ���ᣬŨ��������30.6g��Ȼ��ͨ����ʯ�ң���ʯ������52.8g���������������ͱ���������Ϊ3��2��
���� ��1����Ȼ������Ҫ�ɷ�-��������ʣ�����ȼ��ʱ������Ϊ������̼��ˮ��
��2������Ϊ̼ԭ�ӣ�����Ϊ��ԭ�ӣ�
��3���������ɵ�ˮ�Ͷ�����̼�������ɼ����л����к��е�C��Hԭ�����ʵ��������顢��������ʵ����ֱ�Ϊxmol��ymol������C��Hԭ���غ��з��̼�����
��� �⣺��1����Ȼ������Ҫ�ɷ��Ǽ��飬����ȼ��ʱ������Ϊ������̼��ˮ��������Ȼ������������ȼ�ϵĸĸ���Լ��ٴ�����Ⱦ�����Ƴ��п����������ʴ�Ϊ��C��
��2��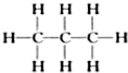 ����Ϊ�����飻
����Ϊ�����飻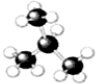 ����Ϊ���춡�飬�ʴ�Ϊ�����飻�춡�飻
����Ϊ���춡�飬�ʴ�Ϊ�����飻�춡�飻
��3��Ũ��������Ϊȼ������ˮ�������������ʵ���=$\frac{30.6g}{18g/mol}$=1.7mol��
��ʯ������Ϊ���ɶ�����̼�������������ʵ���=$\frac{52.8g}{44g/mol}$=1.2mol��
�����顢��������ʵ����ֱ�Ϊxmol��ymol����Hԭ���غ㼰Cԭ���غ㣬��
6x+8y=1.7��2
2x+3y=1.2
���x=0.3 y=0.2��
�������������ͱ���������=0.3mol��0.2mol=3��2��
�ʴ�Ϊ��3��2��
���� ���⿼���˼�������ʺ���;���л������ʽ��ȷ�����Ѷ��еȣ��ؼ��Ǹ��ݷ���ʽȷ�������������أ�ע������ԭ���غ���н������������ѧ�������������������������ѧ����Ӧ��������

| A�� | IA����VIIA��Ԫ�ؼ���γɹ��ۻ���������ӻ����� | |
| B�� | ��������Ԫ�ش����ң�������۴�+1 ������+7 | |
| C�� | ͬ����Ԫ�صļ������ӻ�ԭ��Խǿ������ˮ��Խ����ˮ�� | |
| D�� | ͬ���ڵ��������Ԫ�صĻ��ϼ�Խ�ߣ���ԭ��ʧ��������Խ�� |
| A�� | �ⶨHCl��NaOH��Ӧ���к���ʱ��ÿ��ʵ���Ӧ����3���¶ȣ���������ʼ�¶ȡ�NaOH��ʼ�¶Ⱥͷ�Ӧ����ֹ�¶� | |
| B�� | ��101kPaʱ��1molH2��ȫȼ������ˮ�������ų�285.8kJ������H2��ȼ����Ϊ��H=-285.8kJ•mol-1 | |
| C�� | ��101kPaʱ��1molC������O2��Ӧ����1 mol COʱ���ų�110.5 kJ��������C��ȼ����Ϊ110.5kJ•mol-1 | |
| D�� | ��ϡ��Һ�У�H+��aq��+OH-��aq���TH2O��l����H=-57.3 kJ•mol-1��������0.5 molH2SO4��Ũ��Һ�뺬1 molNaOH����Һ��ϣ��ų�����������57.3kJ |
| A�� | C6H11COOH | B�� | C6H5COOH | C�� | C6H10COOH | D�� | C5H10CH2COOH |
| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �ڢܢ� |
| A�� | Na+��Ba2+��Cl-��SO42- | B�� | K+��Na+��NO3-��OH- | ||
| C�� | H+��NH4+��Fe3+��SO42- | D�� | H+��Cl-��CO32- NO3- |
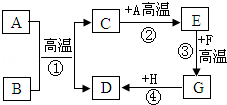 A��H���dz��л�ѧ�г��������ʣ���֪BΪ��ɫ���壬DΪ��ɫ���嵥�ʣ�FΪ��ɫ���壬H��Һ�е�������һ���Σ����ǵ�ת����ϵ��ͼ��ʾ����ش�
A��H���dz��л�ѧ�г��������ʣ���֪BΪ��ɫ���壬DΪ��ɫ���嵥�ʣ�FΪ��ɫ���壬H��Һ�е�������һ���Σ����ǵ�ת����ϵ��ͼ��ʾ����ش� ��
�� ��
�� ��
��