��Ŀ����
����Ŀ����Ʒ������Ϊ�������������������ܼ���ҽҩ�м��塣�ϳɦ�-��Ʒ��(G)��·��֮һ���£�
����E�Ļ�ѧʽΪC8H12O2����ش��������⣺
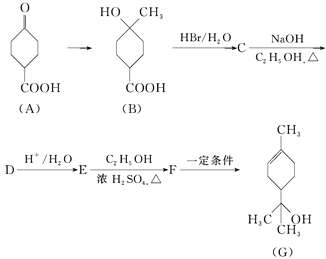
(1)A�к��еĹ�����������________��C�Ľṹ��ʽΪ________��
(2)�ɻ�����C��ȡ������D�ķ�Ӧ����Ϊ________��������������ɻ�����Bֱ����Ũ�����м���Ҳ���Ի�û�����E�����ܿ�����ԣ�����Ϊ����ԭ����_________________________________��
(3)��д���ɻ�����E��ȡ������F�Ļ�ѧ����ʽ��_______________________________________��
(4)������A��������������ͬ���칹����________�֡�
a��Ϊ��״�л�������֧��
b���ܷ���������Ӧ
c���ܺ�̼�����Ʒ�Ӧ��������
(5)����˵����ȷ����________(��д���)��
a��A�ĺ˴Ź���������4�ַ�
b��B����FeCl3��Һ������ɫ��Ӧ
c��G������ԭ�ӿ�����ͬһƽ����
d��G����ʹ��ˮ��ɫ
���𰸡� �ʻ����Ȼ� 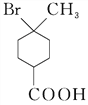 ��ȥ��Ӧ ������B��ŨH2SO4�лᷢ��������Ӧ��Ӱ�����
��ȥ��Ӧ ������B��ŨH2SO4�лᷢ��������Ӧ��Ӱ�����  ��C2H5OH
��C2H5OH![]()
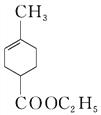 ��H2O 4 ad
��H2O 4 ad
��������A��һ��������ת����B��B��HBr����ȡ����Ӧ����CΪ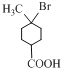 ��C������ȥ��Ӧ����D����D�Ľṹ��ʽΪ��
��C������ȥ��Ӧ����D����D�Ľṹ��ʽΪ��![]() ��D����ˮ�ⷴӦ�õ�E����֪E�Ļ�ѧʽΪ��C8H12O2����E�Ľṹ��ʽΪ
��D����ˮ�ⷴӦ�õ�E����֪E�Ļ�ѧʽΪ��C8H12O2����E�Ľṹ��ʽΪ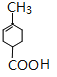 ��E���Ҵ�����������Ӧ����FΪ
��E���Ҵ�����������Ӧ����FΪ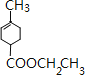 ��F��һ�������·�Ӧ����G��
��F��һ�������·�Ӧ����G��
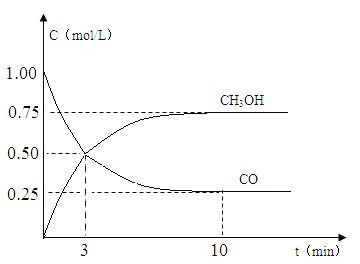
��1������ͼʾ�л���A�Ľṹ��֪��A�к��й�����Ϊ�ʻ����Ȼ���C�Ľṹ��ʽΪ�� ��
��
��2��������C ���������ƵĴ���Һ�з�����ȥ��Ӧ����D
���������ƵĴ���Һ�з�����ȥ��Ӧ����D![]() ��������B�к����ǻ����Ȼ�����Ũ������B�ᷢ��������Ӧ��Ӱ������E�IJ��ʣ�
��������B�к����ǻ����Ȼ�����Ũ������B�ᷢ��������Ӧ��Ӱ������E�IJ��ʣ�
��3���ɻ�����E��ȡ������F�ķ�ѧ����ʽΪ�� +CH3CH2OH
+CH3CH2OH![]()
 +H2O��
+H2O��
��4��a��Ϊ��״�л�������֧���������������л���Ϊ֧��״��b���ܷ���������Ӧ��������к���ȩ����c���ܺ�̼�����Ʒ�Ӧ�������壬˵�������к����Ȼ������A�Ľṹ��ʽ��֪�����л����к���1��ȩ����1���Ȼ���1��̼̼˫��������A��Ϊͬ���칹���������������л����У�HOOCCH=CHCH2CH2CH2CHO��HOOCCH2CH=CHCH2CH2CHO��HOOCCH2CH2CH=CHCH2CHO��HOOCCH2CH2CH2CH=CHCHO���ܹ���4�֣�
��5��a��A�����к���4�ֵ�ЧHԭ�ӣ�������˴Ź���������4�ַ壬��a��ȷ��b��B�����в����������������ǻ�������B������FeCl3��Һ������ɫ��Ӧ����b����c��G�к��м�����Ϊ��������ṹ������G������ԭ�Ӳ�������ͬһƽ���ϣ���c����d��G�к���̼̼˫�����ܹ����巢���ӳɷ�Ӧʹ��ˮ��ɫ����d��ȷ���ʴ�Ϊad��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�