��Ŀ����
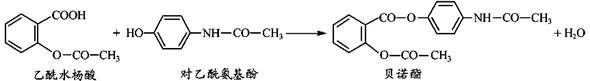
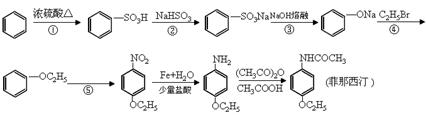
����ѧ��ѡ��5���л���ѧ��������15�֣�ij�����廯����A�ķ���ʽC7H7NO2���˴Ź�����ʾA��3�ֲ�ͬ������H�ҷ����֮��Ϊ3��2��2�����Ա�Ϊԭ�Ϻϳ�A���������Ƶ�F��һ��Ⱦ���м��壩��ת����ϵ���£�(�Լ�a���Լ�bΪ��֪�ж���)
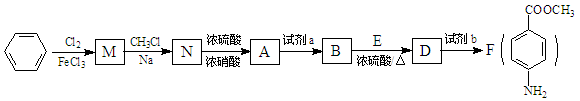
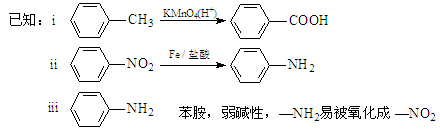
��ش��������⣺
��1������ʯ�ͻ�ѧ��ҵ�ķ�չ��ͨ��ʯ�ͻ�ѧ��ҵ�е�________�ȹ��տ��Ի�÷�������
��2��д��A�Ľṹ��ʽ_______________��
��3��M�Ĺ����ŵ�������_______________ ��A��B�ķ�Ӧ������_______________ ��
��4��������ת�����Լ�b��______��ѡ����ĸ����
A��KMnO4��H+�� B��Fe/���� C��NaOH��Һ
��1 mol ����ܹ���________ mol H+ ������Ӧ��
����ܹ���________ mol H+ ������Ӧ��
��д��M����N�Ļ�ѧ����ʽ_____________��
��5��F�ж���ͬ���칹�壬���������������F��ͬ���칹���� _______________ �֡�
���Ƿ����廯���������������ȡ����������һ��ȡ�����ǡ�NH2��
�ڷ����к��� �ṹ��
�ṹ��
�۷�����������ԭ�Ӳ���ֱ��������
��6��Fˮ����Եõ�E��H��������H��һ�������¾����۷�Ӧ���Ƶø߷�����ά���㷺����ͨѶ�����������������д�������۷�Ӧ�Ļ�ѧ����ʽ��_______________________��


��ش��������⣺
��1������ʯ�ͻ�ѧ��ҵ�ķ�չ��ͨ��ʯ�ͻ�ѧ��ҵ�е�________�ȹ��տ��Ի�÷�������
��2��д��A�Ľṹ��ʽ_______________��
��3��M�Ĺ����ŵ�������_______________ ��A��B�ķ�Ӧ������_______________ ��
��4��������ת�����Լ�b��______��ѡ����ĸ����
A��KMnO4��H+�� B��Fe/���� C��NaOH��Һ
��1 mol
 ����ܹ���________ mol H+ ������Ӧ��
����ܹ���________ mol H+ ������Ӧ����д��M����N�Ļ�ѧ����ʽ_____________��
��5��F�ж���ͬ���칹�壬���������������F��ͬ���칹���� _______________ �֡�
���Ƿ����廯���������������ȡ����������һ��ȡ�����ǡ�NH2��
�ڷ����к���
 �ṹ��
�ṹ�� �۷�����������ԭ�Ӳ���ֱ��������
��6��Fˮ����Եõ�E��H��������H��һ�������¾����۷�Ӧ���Ƶø߷�����ά���㷺����ͨѶ�����������������д�������۷�Ӧ�Ļ�ѧ����ʽ��_______________________��
��15�֣�
��1����������2�֣�
��2��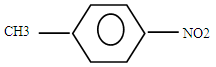 ��2�֣�
��2�֣�
��3����ԭ�ӣ�1�֣� ������Ӧ ��1�֣�
��4�� B ��1�֣� 1��2�֣�
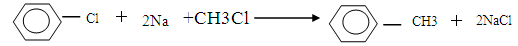 ��2�֣�
��2�֣�
��5��11��2�֣�
��6��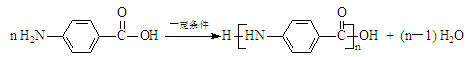 ��2�֣�
��2�֣�
��1����������2�֣�
��2��
 ��2�֣�
��2�֣� ��3����ԭ�ӣ�1�֣� ������Ӧ ��1�֣�
��4�� B ��1�֣� 1��2�֣�
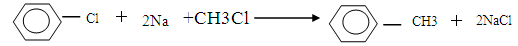 ��2�֣�
��2�֣���5��11��2�֣�
��6��
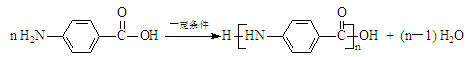 ��2�֣�
��2�֣������������1������������Դ��Ҫ��ú��ʯ�ͣ�ͨ��ʯ�͵Ĵ�������ʹ������������ת��Ϊ��������
��2��������������ȡ����Ӧ����M�ȱ����ȱ���һ�ȼ��鷢��ȡ����Ӧ��N�ױ����ױ���Ũ���ᡢŨ���ᷢ��ȡ����Ӧ���������ױ�A��A��3����ԭ�ӣ�����A�Ƕ������ױ����ṹ��ʽΪ

��3��M���ȱ������Թ����ŵ���������ԭ�ӣ���F�Ľṹ��ʽ�ж�A�е��������ձ䰱���������Ȼ���F�к�������������B�к����Ȼ�����״���Ũ���ᡢ���������·���������Ӧ������A��B�ķ�Ӧ������������Ӧ��
��4������Ϊ�����ױ�����Ϊ����������F�еİ��������һ����ã������Լ�b��Fe/���ᣬ��ѡB��
��1������ֻ��1��H+��Ӧ������1 mol
 ����ܹ���1mol H+ ������Ӧ��
����ܹ���1mol H+ ������Ӧ����M��N��������ȡ����Ӧ������M����N�Ļ�ѧ����ʽΪ
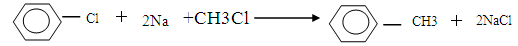 ��
����5�����������֪F��ͬ���칹���г�����֮�����һȡ��������ʽ��4�֣��ֱ���-COOCH3��-OOCCH3��-CH2COOH��--CH2OOCH��������2��ȡ������ͬ���칹�������ڡ��䡢��3�ֽṹ�����Թ���12�֣�ȥ��F����11�֣����Է���������F��ͬ���칹����11�֣�
��6��Fˮ��ü״��Ͷ��������ᣬ������������Է�����ˮ�������ɶ��Ļ��ʣ���ѧ����ʽΪ
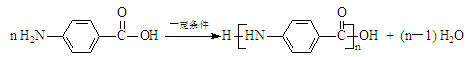

��ϰ��ϵ�д�
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�
�����Ŀ
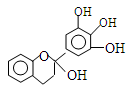
 ������������̼ԭ�ӹ�ƽ��
������������̼ԭ�ӹ�ƽ�� ���Ͷ�ݹ��������
���Ͷ�ݹ�������� ������ijЩ�в�ҩ�еijɷ֣����Ǿ�����ͬ�Ĺ����ţ���Ϊͬϵ��
������ijЩ�в�ҩ�еijɷ֣����Ǿ�����ͬ�Ĺ����ţ���Ϊͬϵ�� ���л������ڵ��ǣ���һ���������ܷ���ȡ������ȥ���ӳɡ���������ԭ�ȷ�Ӧ
���л������ڵ��ǣ���һ���������ܷ���ȡ������ȥ���ӳɡ���������ԭ�ȷ�Ӧ