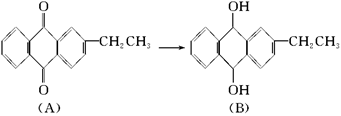
��Ŀ����
(14�֣�ҩ�������͡��һ�ֺϳ�·�����£�
��1��������͡�ķ���ʽ
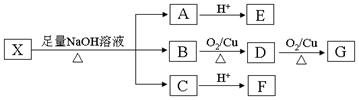
��2����Ӧ�٢ڢܢ����ڱ����Ϸ���ȡ����Ӧ����
��3����Ӧ�������ɵ�������ˮ�� ���ѧʽ��
��4��д����Ӧ�ݵĻ�ѧ����ʽ________________________________________________
��5��������͡���������ˮ��Ļ�ѧ����ʽ��_____________��
��6��������͡��ͬ���칹���У��������������Ĺ��� �֡�
�ٺ�������ֻ�ж�λ����ȡ���� �ڱ����Ϻ��а��� ����ˮ�⣬ˮ������ܷ���������Ӧ��
��7��������͡��ͬ���칹���У���������ֻ�ж�λ����ȡ����������ȡ����������̼ͬԭ�������Һ��Ц�-������ṹ��д������ͬ���칹�����ۺ�߷��Ӳ���Ľṹ��ʽ ��

��1��������͡�ķ���ʽ
��2����Ӧ�٢ڢܢ����ڱ����Ϸ���ȡ����Ӧ����
��3����Ӧ�������ɵ�������ˮ�� ���ѧʽ��
��4��д����Ӧ�ݵĻ�ѧ����ʽ________________________________________________
��5��������͡���������ˮ��Ļ�ѧ����ʽ��_____________��
��6��������͡��ͬ���칹���У��������������Ĺ��� �֡�
�ٺ�������ֻ�ж�λ����ȡ���� �ڱ����Ϻ��а��� ����ˮ�⣬ˮ������ܷ���������Ӧ��
��7��������͡��ͬ���칹���У���������ֻ�ж�λ����ȡ����������ȡ����������̼ͬԭ�������Һ��Ц�-������ṹ��д������ͬ���칹�����ۺ�߷��Ӳ���Ľṹ��ʽ ��
(1) C10H13NO2 ��(2) �٢���(3) SO2 ��
(4)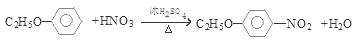
(5) ��
��
(6) 5��(7) ��
��
(4)
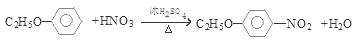
(5)
 ��
��(6) 5��(7)
 ��
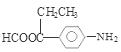
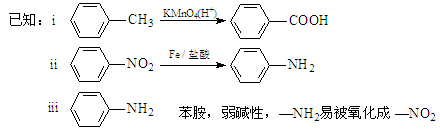
�������������1���ɷ�����͡�Ľṹ��ʽ��֪�����ʽ��C10H13NO2 ����2����Ӧ���DZ��Ļǻ���Ӧ������ȡ����Ӧ����ȷ������������ʽ�εĸ��ֽⷴӦ��������ȡ����Ӧ�����Dz��Ƿ����ڱ����ϣ������DZ�����������Ӧ������ȡ����Ӧ����ȷ���������Щ�ڱ����Ϸ���ȡ����Ӧ���Ǣ٢ݣ���3����Ӧ�������ɵ������б������ƺ�����ˮ��SO2 ����4����Ӧ�ݵĻ�ѧ����ʽ
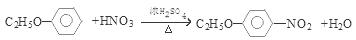 ����5��������͡
����5��������͡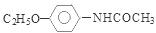 �����к����ļ������������ˮ��õ�
�����к����ļ������������ˮ��õ�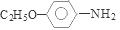 ��CH3COOH����Ӧ�Ļ�ѧ����ʽ�ǣ�
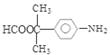
��CH3COOH����Ӧ�Ļ�ѧ����ʽ�ǣ�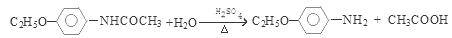 ����6�����������ķ�����͡��ͬ���칹�������֡����Ƿֱ��ǣ�
����6�����������ķ�����͡��ͬ���칹�������֡����Ƿֱ��ǣ�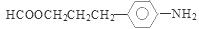 ��
��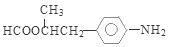 ��
��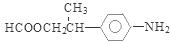 ��
�� ��
�� ��7��������͡��ͬ���칹���У���������ֻ�ж�λ����ȡ����������ȡ����������̼ͬԭ�������Һ��Ц�-������ṹ����ṹ��
��7��������͡��ͬ���칹���У���������ֻ�ж�λ����ȡ����������ȡ����������̼ͬԭ�������Һ��Ц�-������ṹ����ṹ��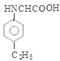 ������ͬ���칹�����ۺ�߷��Ӳ���Ľṹ��ʽ��
������ͬ���칹�����ۺ�߷��Ӳ���Ľṹ��ʽ��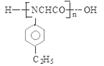 ��
��
��ϰ��ϵ�д�
�����Ŀ
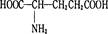 ͬ���칹���кܶ࣬���к���һ������һ��������һ���Ȼ���ͬ���칹�干�� ( )
ͬ���칹���кܶ࣬���к���һ������һ��������һ���Ȼ���ͬ���칹�干�� ( )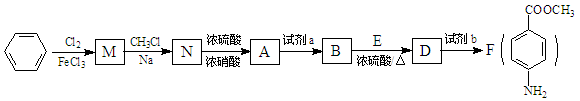
 ����ܹ���________ mol H+ ������Ӧ��
����ܹ���________ mol H+ ������Ӧ�� �ṹ��
�ṹ�� 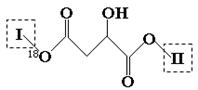

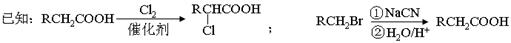
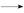 RCH=C(COOH)2+H2O
RCH=C(COOH)2+H2O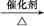 RCH=CHCOOH+CO2������ش��������⡣
RCH=CHCOOH+CO2������ش��������⡣
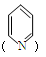 �һ���ֻ��2��ȡ������
�һ���ֻ��2��ȡ������ ��
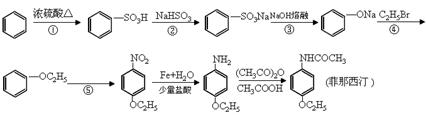
�� Ϊԭ�Ϻϳ������N�����������ǰ���
Ϊԭ�Ϻϳ������N�����������ǰ���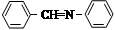 ������������ϳ�·�ߣ� ��
������������ϳ�·�ߣ� ��