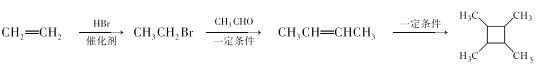
��Ŀ����
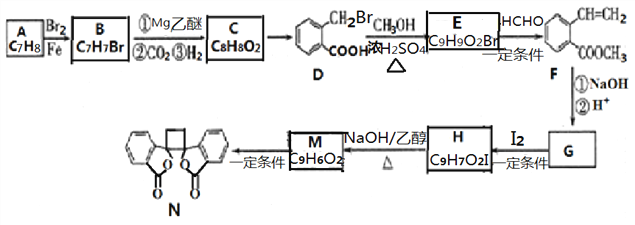
����Ŀ�����п������õİ�ͷ����������N�ĺϳ�·������ͼ��ʾ:
��֪: i�� 
ii�� 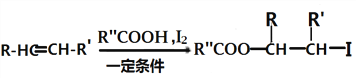
iii��  (����R��R��R�������⡢�������)
(����R��R��R�������⡢�������)
�ش���������:
��1������B��������_______������D���������ŵ�������_______ ��
��2������C�Ľṹ��ʽ��_______����C��D�ķ�Ӧ����Ϊ_______��
��3����H����M�Ļ�ѧ����ʽΪ_______��
��4��F��ͬ���칹���У�����������������______��(����˳���칹)��д���˴Ź���������6��塢�����Ϊ1:1:1:2:2:3��һ���л���Ľṹ��ʽ____________��
����:�ٷ����к��б���,�ұ�����������ȡ�����ڴ���˳���칹
������̼�����Ʒ�Ӧ����CO2
��5��д������ϩ����ȩΪԭ���Ʊ������� �ĺϳ�·�� (�����Լ���ѡ)��________
�ĺϳ�·�� (�����Լ���ѡ)��________
���𰸡� 2-��ױ���������ױ��� ��ԭ�ӡ��Ȼ� 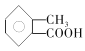 ȡ����Ӧ
ȡ����Ӧ 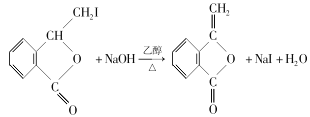 12
12 ![]() ��
��![]()
����������A�ķ���ʽ�Ա�D�Ľṹ��ʽ��֪A�к��б�����Ϊ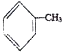 ������C��D�ķ���ʽ��IJ���֪��CΪ
������C��D�ķ���ʽ��IJ���֪��CΪ![]() ������B����C�ķ�Ӧ������֪��BΪ
������B����C�ķ�Ӧ������֪��BΪ![]() ��CΪ
��CΪ![]() ��D��״�����������Ӧ����E��EΪ
��D��״�����������Ӧ����E��EΪ![]() ��Fˮ����ữ����G��GΪ
��Fˮ����ữ����G��GΪ![]() ������Ϣii��֪HΪ
������Ϣii��֪HΪ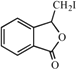 ��H�Ľṹ��Ϸ�Ӧ��������֪��MΪ
��H�Ľṹ��Ϸ�Ӧ��������֪��MΪ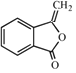 ��
��
(1)BΪ![]() ������Ϊ����ױ�������D(
������Ϊ����ױ�������D(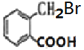 )��������������ԭ�ӡ��Ȼ����ʴ�Ϊ������ױ�����ԭ�ӡ��Ȼ���
)��������������ԭ�ӡ��Ȼ����ʴ�Ϊ������ױ�����ԭ�ӡ��Ȼ���
(2)������������������C�Ľṹ��ʽ![]() Ϊ��C(
Ϊ��C(![]() )�������ϵ������Ӧ����D(
)�������ϵ������Ӧ����D( )���ʴ�Ϊ��
)���ʴ�Ϊ��![]() ��ȡ����Ӧ��
��ȡ����Ӧ��
(3)H����±��ԭ�ӵ���ȥ��Ӧ����M����Ӧ�Ļ�ѧ����ʽΪ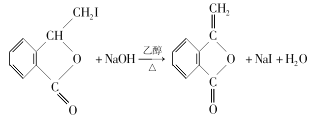 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
(4)FΪ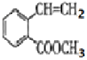 )���ٷ����к��б������ұ�����������ȡ�������ڴ���˳���칹��˵��̼̼˫������̼ԭ�������ӵ�2�������Dz�ͬ�ģ�������̼�����Ʒ�Ӧ����CO2��˵�������Ȼ�������������F��ͬ���칹���У�����ȡ�������������COOH����CH=CHCH3����CH3����CH=CHCOOH���ڱ����Ͼ�����λ����λ����λ3��λ�ù�ϵ������˳���칹����2��3��2=12�֣����к˴Ź���������6��塢�����Ϊ1:1:1:2:2:3����
)���ٷ����к��б������ұ�����������ȡ�������ڴ���˳���칹��˵��̼̼˫������̼ԭ�������ӵ�2�������Dz�ͬ�ģ�������̼�����Ʒ�Ӧ����CO2��˵�������Ȼ�������������F��ͬ���칹���У�����ȡ�������������COOH����CH=CHCH3����CH3����CH=CHCOOH���ڱ����Ͼ�����λ����λ����λ3��λ�ù�ϵ������˳���칹����2��3��2=12�֣����к˴Ź���������6��塢�����Ϊ1:1:1:2:2:3����![]() ��
��![]() ���ʴ�Ϊ��12��
���ʴ�Ϊ��12��![]() ��
��![]() ��
��
(5)����ϩΪ��ʼԭ�Ϻϳ�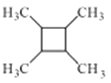 ��Ӧ������CH3CH=CHCH3��������ȩ��CH3CH2Br���ɣ���ϩ�����������飬��Ӧ������Ϊ
��Ӧ������CH3CH=CHCH3��������ȩ��CH3CH2Br���ɣ���ϩ�����������飬��Ӧ������Ϊ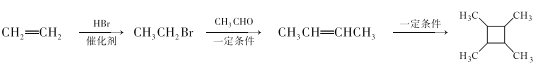 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��

����Ŀ��[��ѧһһѡ��3�����ʽṹ������]̼��Ԫ�صĵ��ʺͻ������ڻ�����ҽҩ�����ϵ��������Ź㷺��Ӧ�á��ش��������⣺
��1��������Ҫ�뵼����ϣ���̬Geԭ���У��������ռ������ܼ��ķ�����__________�����ܼ��ĵ���������ͼΪ______________������Ge�����ṹ����ʯ���ƣ��ʵ�Ӳ���࣬�е�2830�棬�ྦྷ������__________���塣
��2����(CH3)3C+���л��ϳ���Ҫ�м��壬���м�����̼ԭ���ӻ���ʽΪ______________��(CH3)3C+��̼�Ǽܵļ��ι���Ϊ____________________��
������Ǧ�ж��ɵ�ע�������Ƹƣ�ʹPb2+ת��Ϊ������Ǧ�Ρ�����˵����ȷ����_______�����ţ�
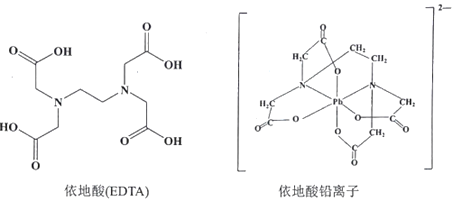
A.�γ�������Ǧ��������n(Pb2+):n(EDTA)=1:4
B.�������и�Ԫ�صĵ縺�ԴӴ�С��˳��ΪO>N>C>H
C.������Ǧ���к������Ӽ�����λ��
D.������������õ�ˮ��������������Ӽ����γ����
��3���±��г��˼�������̼���ε��ȷֽ��¶Ⱥ������Ӱ뾶��
̼���� | MgCO3 | CaCO3 | SrCO3 | BaCO3 |
�ȷֽ��¶�/�� | 402 | 900 | 1172 | 1360 |
�����Ӱ뾶/pm | 66 | 99 | 112 | 135 |
��������̼����ͬ�������ϵ��µ��ȷֽ��¶������ߣ�ԭ���ǣ�__________________________��
��4���л�±��Ǧ������ж��صĹ�����ܣ���ͼΪ�侧���ṹʾ��ͼ��
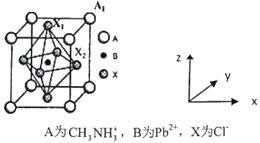
�����þ����ı߳�Ϊanm����Cl-�����̾�����____________________��
���ڸþ�������һ�ֱ��﷽ʽ�У���ͼ��Pb2+���ڶ���λ�ã���Cl-����_________λ�á�ԭ���������BΪ(0,0,0)��A1Ϊ(1/2,1/2,1/2)����X2Ϊ_________________��