��Ŀ����
��12�֣�����ѧ������ѧ�뼼����
�ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ���壬��ͼ�ǹ�ҵ�ϳɰ��ļ�Ҫ����ʾ��ͼ��
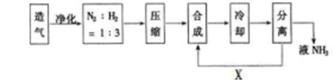
��1���ϳɰ���ԭ������������CO2�����ʣ��ڽ���ϳ���֮ǰ�辻������ԭ����_______ ��
��2����ҵ�Ϻϳɰ�ѡ������������ǣ�����Ϊ���Ĵ�����_______��________��
��3����X·�߽���ϳ�����������_______��������Ƶ�������________��
��4��Ŀǰ��ҵ�����������õ���Ҫ������_______����
�ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ���壬��ͼ�ǹ�ҵ�ϳɰ��ļ�Ҫ����ʾ��ͼ��

��1���ϳɰ���ԭ������������CO2�����ʣ��ڽ���ϳ���֮ǰ�辻������ԭ����_______ ��
��2����ҵ�Ϻϳɰ�ѡ������������ǣ�����Ϊ���Ĵ�����_______��________��
��3����X·�߽���ϳ�����������_______��������Ƶ�������________��
��4��Ŀǰ��ҵ�����������õ���Ҫ������_______����
��12�֣�
��1����ֹ����ʹ�ϳɰ����õĴ������ж�����2�֣�
��2��400~500�� ��2�֣� 10~30Mpa��2�֣�
��3��N2��H2��2�֣� δ��Ӧ�ĵ���������ѭ������ϳ�����ʹ�����������õ�������ã�2�֣�
��4��������������������������2�֣�
��1����ֹ����ʹ�ϳɰ����õĴ������ж�����2�֣�
��2��400~500�� ��2�֣� 10~30Mpa��2�֣�
��3��N2��H2��2�֣� δ��Ӧ�ĵ���������ѭ������ϳ�����ʹ�����������õ�������ã�2�֣�
��4��������������������������2�֣�
�����������1��ԭ������������CO��NH3�����ʣ��ڽ���ϳ���֮ǰΪ��ֹ�����ж�������о�����
��2��ѡ��400�桫500�棬��Ӧ���ʺ�ƽ��ת���ʶ��ϸߣ����¶ȹ��ߣ�ת���ʷ������ͣ���ѹ�£�ƽ��ת���ʽϸߣ�������ѹǿ��������豸��ۺͺ������ӣ��ʴ�Ϊ��400��500�棻10��30Mpa��
��3���ϳɰ���ҵ�У����ð���Һ���������N2��H2ѭ��ʹ�ã���ʹƽ��������Ӧ�����ƶ��������IJ�������δ��Ӧ�ĵ���������ѭ������ϳ�����ʹ�����������õ�������ã������ԭ�ϵ������ʡ�
��4����ҵ�����������õ���Ҫ�����ǰ�����������

��ϰ��ϵ�д�
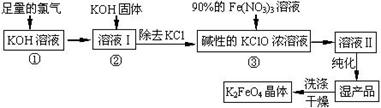
�����Ŀ

 CaCO3��+2NaOH
CaCO3��+2NaOH 2C2H5OH+2CO2��
2C2H5OH+2CO2�� CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O CaCl2+2Cl2��+2H2O
CaCl2+2Cl2��+2H2O