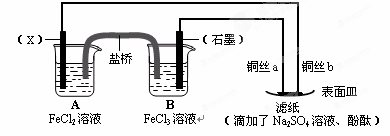
��Ŀ����
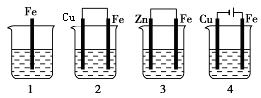
��.�õ��߽�A��B��װ�õ��ĸ��缫�������ӣ�ʹa������ͭ���ش��й����⡣
��1�����߽�����A��B����ʱ��Zn�� ��Cu�� ���a����b����
��2������A��Cu�������ĵ缫��ӦΪ ��
��3��Bװ�ý� ����Һ�е�NO3-��_____���ƶ����a����b������
��4����b���۲쵽����ɫ��ζ���ݲ���, ����һ��ʱ���ֹͣ��Ӧ��������ȣ���Һ��pHֵ�� ������ߡ��������͡����䡱��������һ������ ���ѧʽ������Һ�ָܻ����뷴Ӧǰ��ȫһ�¡�����Ӧһ��ʱ������Һ��Cu2+Ũ��û�������½������ܵ�ԭ���ǣ� ��
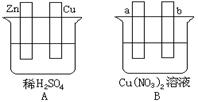
��.���˵�����û��ϴ�ɾ��������⡣�ñ�Ҫ�����ֺ��йػ�ѧ����ʽ˵������������γɵ�
��

��1�����߽�����A��B����ʱ��Zn�� ��Cu�� ���a����b����
��2������A��Cu�������ĵ缫��ӦΪ ��
��3��Bװ�ý� ����Һ�е�NO3-��_____���ƶ����a����b������
��4����b���۲쵽����ɫ��ζ���ݲ���, ����һ��ʱ���ֹͣ��Ӧ��������ȣ���Һ��pHֵ�� ������ߡ��������͡����䡱��������һ������ ���ѧʽ������Һ�ָܻ����뷴Ӧǰ��ȫһ�¡�����Ӧһ��ʱ������Һ��Cu2+Ũ��û�������½������ܵ�ԭ���ǣ� ��
��.���˵�����û��ϴ�ɾ��������⡣�ñ�Ҫ�����ֺ��йػ�ѧ����ʽ˵������������γɵ�
��
��.��1��a��b ��2��2H+ + 2e- = H2����3�����أ�b��������
��4�����ͣ�CuO b������ΪCu
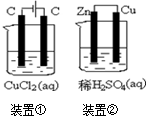
��.��������������ģ������е�Fe��C�ͳ���δϴ����ʳ��ˮ����ԭ��أ�1�֣���ʹ�������绯ѧ��ʴ����Fe(OH)2�� Fe(OH)2�ٱ�������Fe(OH)3��Fe(OH)3����ʧˮ�õ����⡣������2Fe - 4e- = 2Fe2+��������O2 + 2H2O +4e- = 4OH-��
4Fe(OH) +O2 +2H2O = 4Fe(OH)3
��4�����ͣ�CuO b������ΪCu
��.��������������ģ������е�Fe��C�ͳ���δϴ����ʳ��ˮ����ԭ��أ�1�֣���ʹ�������绯ѧ��ʴ����Fe(OH)2�� Fe(OH)2�ٱ�������Fe(OH)3��Fe(OH)3����ʧˮ�õ����⡣������2Fe - 4e- = 2Fe2+��������O2 + 2H2O +4e- = 4OH-��
4Fe(OH) +O2 +2H2O = 4Fe(OH)3
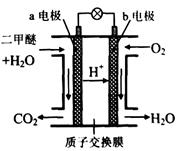
���������I.A���γ�ԭ��أ�Zn��������Cu��������ʹa������ͭ��a��Ϊ����������a��Zn���ӣ�b��Cu���ӡ�A��Cu�������ķ�ӦΪ2H++2e-=H2����Zn�������ķ�ӦΪZn-2e-=Zn2+��Bװ�ýе��أ���Һ��NO3-�������ƶ����������ͭ��Һ����ͭ���������������Һ������ǿ��pH��С������������������������ͭ����������ͭ��ʹ��ҺŨ�Ȼָ�����ӦǰŨ�ȣ�����Ӧһ��ʱ���Cu2+Ũ��û�������½���˵�������ܽ�Cu����Cu2+��II.������Ļ���������̿��ʳ��ˮ�γ�ԭ��أ�FeΪ������Fe-2e-=Fe2+��̿Ϊ�������缫��ӦΪO2+2H2O+4e-=4OH-���ܷ�ӦΪ2Fe+O2+2H2O=2Fe(OH)2��Fe(OH)2������ΪFe(OH)3��Fe(OH)3ʧˮ����Fe2O3��xH2O��
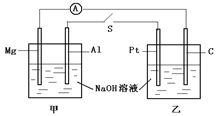
�����������绯��ʴ�����֣�������ʴ�����ⸯʴ�����������Һ���Խ�ǿʱ���������ⸯʴ�����������Һ�����Ի����ʱ����������ʴ��

��ϰ��ϵ�д�
�����Ŀ
 ת��Ϊ
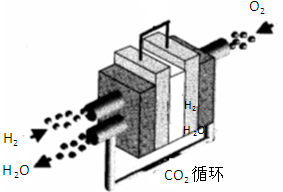
ת��Ϊ 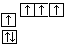 ʱ��̼ԭ��Ҫ����绷����������
ʱ��̼ԭ��Ҫ����绷����������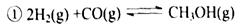


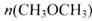 =_________mol��
=_________mol��