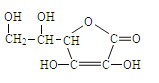
��Ŀ����
����Ŀ����ʵ����ģ�ҵ����̼���ƣ�һ���¶��£���һ��������NaCl��Һ��ͨ�백���ﵽ���ͺ��ٲ���ͨ��CO2��һ��ʱ����ֳ��������˵õ�NaHCO3���塣
��1���ù��̵Ļ�ѧ����ʽ�� ��
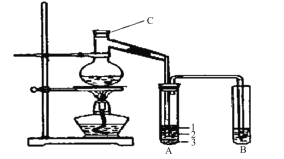
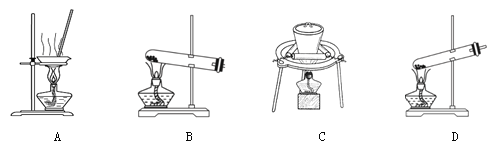
��2������NaHCO3�õ�������Ʒ��ʵ���ҿɽ��д˲�����װ���� ��
��3���õ��Ĵ�����Ʒ��������NaHCO3��NaCl�����ʵ����֤��Ʒ�и�����֡�
�Լ���������ѡ�ã��Թܡ���ͷ�ιܡ�����װ�á�Ba��NO3��2��Һ��NaOH��Һ��AgNO3��Һ������ʯ��ˮ��ϡ���ᡢϡ���ᡢϡ���ᡣ
����һ��ȡ������Ʒ���Թ��У�����������ˮ�����ܽ⡣���Թ��м��� ���۲죻 | ������Һ�в���������ɫ������ ���ۣ� �� |
�������������һ����Һ���ˣ�ȡ��Һ���Թ���B�У����� �� ���۲죻 | ���� �� ���ۣ���Ʒ�к���NaHCO3�� |
�������������������Һ���ˣ�ȡ��Һ���Թ�C�У� ���۲졣 | ���� �� ���ۣ� �� |
���𰸡���1�� NaCl + NH3 + CO2 + H2O = NaHCO3 ��+ NH4Cl ��2�֣�û�г������ſ�1����
��2��BC ��3����
��3��
����һ��ȡ������Ʒ���Թ��У�������������ˮ�����ܽ⣺���Թ��м��� ������Ba��NO3��2��Һ�����۲� ��2���� | ������Һ�в���������ɫ������ ���ۣ������к���Na2CO3����1���� |
�������������һ����Һ���ˣ�ȡ��Һ���Թ�B�У����� ������NaOH��Һ�� ���۲���1���� | ������������ɫ�������� ����1���� ���ۣ������к���NaHCO3�� |
�������� �����������Һ���˺�ȡ��Һ���Թ�C�У��ȼ���������ϡ���ᣬ�ټ�������AgNO3��Һ�����۲���2���� | ������������ɫ�������� �� ���ۣ������к���NaCl ����1���� |
��������
�����������1����ӦΪ�����Ƽԭ�����ɷ�Ӧ����������֪��Ӧ�ķ���ʽΪNaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl��
��2�����ȹ��壬�����������Թ��н��У���BC���ϣ�D�Թܿ�Ӧ������б����ֹ�Թ�ը�ѣ��ʴ�ΪBC��
��3��������Ʒ����Na2CO3������NaHCO3��NaCl������NaHCO3���ù�����Ba��NO3��2��Һ�ȳ�ȥNa2CO3��Ȼ�����NaOH��Һ�������ɳ�������˵������NaHCO3��ԭ����̼�����ƺ��������Ʒ�Ӧ����̼���ƣ�̼���ƺ����ᱵ��Ӧ����̼�ᱵ���������˺���������ữ�������������ɼ���NaCl��