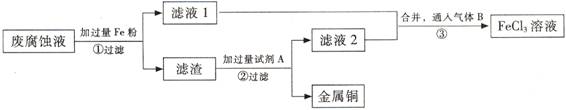
��Ŀ����
(I)���ӹ�ҵ����30����FeCl3����Һ��ʴ���ھ�Ե���ϵ�ͭ��������ӡˢ��·�塣��FeCl3��Һ����ʴҺ��Cu��Ӧ����CuCl2��FeCl2��
(1)д���÷�Ӧ�Ļ�ѧ����ʽ ��
(2)������Һ��Fe3+���ڵ��Լ���
(��)ӡˢ��·�ķϸ�ʴҺ���д���CuCl2��FeCl2��FeCl3�������ŷŻ���ɻ�����Ⱦ����Դ���˷ѡ�ͨ���������̿ɴӸ÷�Һ�л���ͭ���������Ļ�����ȫ��ת��ΪFeCl3��Һ����Ϊ��ʴҺԭ��ѭ��ʹ�á�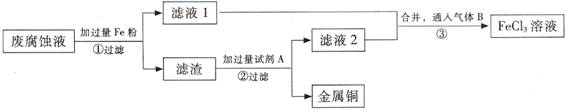 (3) ������з�����Ӧ�����ӷ���ʽ
(3) ������з�����Ӧ�����ӷ���ʽ
(4)��������Ҫ�ɷ��� �� (�ѧʽ)��
(5)Ϊ�˳�ȥ�����е����ʵõ�ͭ���������Լ�A�� (�ѧʽ)��
��8�֣� (1)2FeCl3+Cu==2FeCl2��CuCl2 (2��) (2)KSCN��Һ(1��)
(3)2Fe3++Fe��3Fe2+ (1��) Fe+Cu2+��Fe2++Cu (1��)
(4)Cu��Fe (2��) (5)HCI (1��)
���������������1��FeCl3��Һ����ʴҺ����Cu��Ӧ����CuCl2��FeCl2����Ӧ�Ļ�ѧ����ʽ��2FeCl3+Cu��2FeCl2��CuCl2��
��2���������ܺ�KSCN��Һ��Ӧ����Һ�Ժ�ɫ�����Լ�����Һ��Fe3+���ڵ��Լ���KSCN��Һ��
��3�������ӡ�ͭ���Ӷ��ܺ�����Ӧ����Ӧ�����ӷ���ʽ�ֱ���2Fe3++Fe��3Fe2+��Fe+Cu2+��Fe2++Cu��
��4���������ǹ����ģ�������������Ҫ�ɷ�������ͭ��
��5�����ǻ��õĽ������ܺ������ϡ���ᷴӦ����������Ҫ�õ��Ȼ�����Һ������Ϊ�˳�ȥ�����е����ʵõ�ͭ���������Լ�A�����������ϡ���ᡣ
���㣺���������仯��������ʡ������ӵļ��顢���ʵķ�����ᴿ
�������������е��Ѷȵ����⣬����ע�ػ�����������˫�顣�����ۺ���ǿ��ע��������ѵ��������������ѧ������˼ά�����淶�Ĵ���������

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
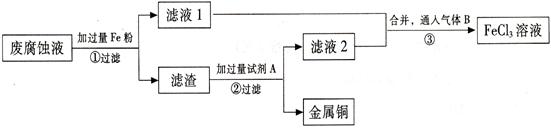
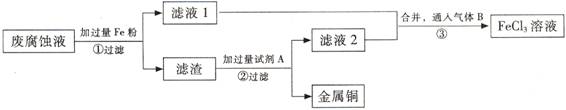 (3) ������з�����Ӧ�����ӷ���ʽ
(3) ������з�����Ӧ�����ӷ���ʽ