Ő‚ńŅńŕ»›
(I) ĶÁ◊”Ļ§“Ķ≥£”√30£•ĶńFeCl3»‹“ļłĮ ī∑ů‘ŕĺÝ‘ĶįŚ…ŌĶńÕ≠≤≠£¨÷∆‘ž”°ňĘĶÁ¬∑įŚ°£”√FeCl3»‹“ļ◊ŲłĮ ī“ļ”ŽCu∑ī”¶…ķ≥…CuCl2ļÕFeCl2°£
(1) –ī≥Ųł√∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺ °£
(2) ľž—ť»‹“ļ÷–Fe3+īś‘ŕĶń ‘ľŃ « £¨÷§√ųFe3+īś‘ŕĶńŌ÷Ōů « °£
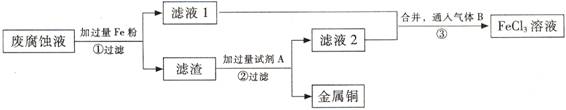
(ĘÚ) ”°ňĘĶÁ¬∑Ķń∑ŌłĮ ī“ļļ¨”–īůŃŅCuCl2°ĘFeCl2°ĘFeCl3£¨»ő“‚ŇŇ∑ŇĽŠ‘ž≥…Ľ∑ĺ≥őŘ»ĺľį◊ ‘īĶńņň∑—°£Õ®ĻżŌ¬Ń–Ńų≥ŐŅ…ī”ł√∑Ō“ļ÷–Ľō ’Õ≠£¨≤ĘĹęŐķĶńĽĮļŌőÔ»ę≤Ņ◊™ĽĮő™FeCl3»‹“ļ£¨◊ųő™łĮ ī“ļ‘≠ŃŌ—≠Ľ∑ Ļ”√°£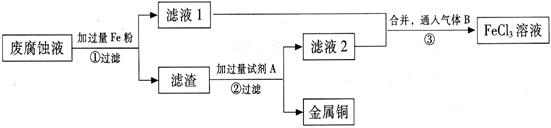
ĘŇ ≤Ĺ÷ŤĘŔ÷–∑Ę…ķ∑ī”¶ĶńņŽ◊”∑Ĺ≥Ő Ĺ °£
(2) ≤Ĺ÷ŤĘŕ–Ťľ”»ŽĶń ‘ľŃA « (ŐÓĽĮ—ß Ĺ)°£
(3) ≤Ĺ÷ŤĘŘÕ®»ŽĶń∆ÝŐŚB « (ŐÓĽĮ—ß Ĺ)£¨–ī≥Ųł√∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺ °£
(4) ő™≤‚∂®¬ň‘Ł÷–Õ≠Ķń÷ ŃŅ∑÷ ż£¨»°50gĶń¬ň‘Ł”Ž◊„ŃŅĶń ‘ľŃA∑ī”¶£¨Ķ√ĶĹ5.6L∆ÝŐŚ£®ĪÍ◊ľ◊īŅŲŌ¬£©£¨ ‘ľ∆ň„¬ň‘Ł÷–Õ≠Ķń÷ ŃŅ∑÷ ż°£
£®16∑÷£©
(I) (1)2FeCl3+Cu==2FeCl2£ęCuCl2 (2∑÷) [ĽĮ—ß ĹīŪ≤ĽłÝ∑÷]
(2)KSCN»‹“ļ(1∑÷)£¨ »‹“ļĪš≥…—™ļž…ę(1∑÷)
(II) (1)2Fe3++Fe£Ĺ3Fe2+ (2∑÷) Fe+Cu2+£ĹFe2++Cu (2∑÷) [ĽĮ—ß ĹīŪ≤ĽłÝ∑÷]
(2) HCl (1∑÷)
(3) C12 (1∑÷) 2FeCl2+C12£Ĺ2FeCl3 (2∑÷) [ĽĮ—ß ĹīŪ≤ĽłÝ∑÷]
(4) 72% £®4∑÷£©£®įīĹ‚Ő‚≤Ĺ÷ŤłÝ∑÷£©
∑Ĺ≥Ő Ĺ’ľ1∑÷
ň„≥ŲŐķĶń÷ ŃŅ£®ĽÚőÔ÷ ĶńŃŅ’ľ1∑÷£©’ľ2∑÷
ň„≥ŲÕ≠Ķń÷ ŃŅ∑÷ ż’ľ1∑÷
Ĺ‚őŲ ‘Ő‚∑÷őŲ£ļ(I) (1)łýĺ›Ő‚÷–ŐŠĻ©Ķń∑ī”¶őÔļÕ…ķ≥…őÔ£¨Ļ ∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺő™2FeCl3+Cu==2FeCl2£ęCuCl2°£
(2)ľž—ťFe3+ņŻ”√∆šŐō’ų∑ī”¶£¨Ļ ‘ľŃ «KSCN»‹“ļ£¨Ō÷Ōů «»‹“ļĪš≥…—™ļž…ę°£
(ĘÚ) ĘŇFe∑ŘĽŠļÕCuCl2°ĘFeCl3∑ī”¶£¨Ļ ņŽ◊”∑Ĺ≥Ő Ĺő™2Fe3++Fe£Ĺ3Fe2+ Fe+Cu2+£ĹFe2++Cu°£
(2)ĻżŃŅĶńFe∑Ř‘ŕ¬ň‘Ł÷–£¨ľ”»ŽHCl Ļ∆š∑ī”¶£¨Ļ ľ”»ŽĶń ‘ľŃA «HCl°£
(3)¬ň“ļ1°Ę¬ň“ļ2÷–∂ľ «Fe2+ņŽ◊”£¨ľ”»Ž¬»∆Ý Ļ∆š∑ī”¶…ķ≥…Fe3+£¨Ļ Õ®»ŽĶń∆ÝŐŚB «C12£Ľ∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺő™2FeCl2+C12£Ĺ2FeCl3°£
(4)“ņĺ›∑ī”¶∑Ĺ≥Ő ĹFe+Cu2+£ĹFe2++Cu Fe+2HCl= FeCl2+H2°Łľ∆ň„°£
ŅľĶ„£ļŐķ°ĘŐķĶńĽĮļŌőÔ°ĘÕ≠÷ģľšĶń∑ī”¶
Ķ„∆ņ£ļĪĺŐ‚ «”–Ļō Ķ—ť∑ĹįłĶń…Ťľ∆ļÕőřĽķ∑ī”¶ĶńŅľ≤ť£¨“™«ů—ß…ķ žŌ§ňý Ķ—ťĶńńŕ»›ľį‘≠ņŪ£¨ń‹ĻĽŅľ≤ťÕ¨—ß√«ĹÝ––∑÷őŲő Ő‚°ĘĹ‚ĺŲő Ő‚Ķńń‹Ń¶°£

 Õ¨≤ĹŃ∑Ōį«ŅĽĮÕō’ĻŌĶŃ–īūįł
Õ¨≤ĹŃ∑Ōį«ŅĽĮÕō’ĻŌĶŃ–īūįł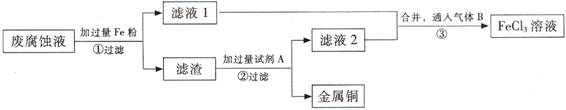 (3) ≤Ĺ÷ŤĘŔ÷–∑Ę…ķ∑ī”¶ĶńņŽ◊”∑Ĺ≥Ő Ĺ
(3) ≤Ĺ÷ŤĘŔ÷–∑Ę…ķ∑ī”¶ĶńņŽ◊”∑Ĺ≥Ő Ĺ 
 (3) ≤Ĺ÷ŤĘŔ÷–∑Ę…ķ∑ī”¶ĶńņŽ◊”∑Ĺ≥Ő Ĺ
(3) ≤Ĺ÷ŤĘŔ÷–∑Ę…ķ∑ī”¶ĶńņŽ◊”∑Ĺ≥Ő Ĺ 