ΧβΡΩΡΎ»ί
14Θ°œ¬Ν–”–ΙΊάκΉ”ΖΫ≥Χ Ϋ ι–¥’ΐ»ΖΒΡ «Θ®ΓΓΓΓΘ©| AΘ° | ¬Ν»ή”Ύ«β―θΜ·ΡΤ»ή“ΚΘΚAl+2OH-+H2O®TAlO2-+2H2Γϋ | |
| BΘ° | Ά≠Τ§ΆΕ»κ…ΌΝΩFeCl3»ή“Κ÷–ΘΚ3Cu+2Fe3+®T2Fe+3Cu2+ | |
| CΘ° | CaΘ®HCO3Θ©2»ή“Κ”κΉψΝΩ≥Έ«ε ·Μ“Υ°ΜλΚœΘΚCa2++HCO3-+OH-®TCaCO3Γΐ+H2O | |
| DΘ° | ”ΟΧζΑτΉςΒγΦΪΒγΫβCuSO4»ή“ΚΘΚ2Cu2++2H2O$\frac{\underline{\;ΒγΫβ\;}}{\;}$2CuΓΐ+O2Γϋ+4H+ |
Ζ÷Έω AΘ°ΒγΉ”ΓΔΒγΚ…≤Μ ΊΚψΘΜ
BΘ°Ζ¥”Π…ζ≥…¬»Μ·Ά≠ΓΔ¬»Μ·―«ΧζΘΜ
CΘ°Ζ¥”Π…ζ≥…ΧΦΥαΗΤΚΆΥ°ΘΜ
DΘ°―τΦΪFe ß»ΞΒγΉ”Θ§“θΦΪΆ≠άκΉ”ΒΟΒΫΒγΉ”Θ°
Ϋβ¥π ΫβΘΚAΘ°¬Ν»ή”Ύ«β―θΜ·ΡΤ»ή“ΚΒΡάκΉ”Ζ¥”ΠΈΣ2Al+2OH-+2H2O®T2AlO2-+3H2ΓϋΘ§Ι A¥μΈσΘΜ
BΘ°Ά≠Τ§ΆΕ»κ…ΌΝΩFeCl3»ή“Κ÷–ΒΡάκΉ”Ζ¥”ΠΈΣCu+2Fe3+®T2Fe2++Cu2+Θ§Ι B¥μΈσΘΜ
CΘ°CaΘ®HCO3Θ©2»ή“Κ”κΉψΝΩ≥Έ«ε ·Μ“Υ°ΜλΚœΒΡάκΉ”Ζ¥”ΠΈΣCa2++HCO3-+OH-®TCaCO3Γΐ+H2OΘ§Ι C’ΐ»ΖΘΜ
DΘ°”ΟΧζΑτΉςΒγΦΪΒγΫβCuSO4»ή“ΚΒΡάκΉ”Ζ¥”ΠΈΣCu2++Fe$\frac{\underline{\;ΒγΫβ\;}}{\;}$Fe2++CuΘ§Ι D¥μΈσΘΜ
Ι ―ΓCΘ°
ΒψΤά ±ΨΧβΩΦ≤ιάκΉ”Ζ¥ΖΫ≥Χ Ϋ ι–¥ΒΡ’ΐΈσ≈–ΕœΘ§ΈΣΗΏΤΒΩΦΒψΘ§Α―Έ’ΖΔ…ζΒΡΖ¥”ΠΦΑάκΉ”Ζ¥”ΠΒΡ ι–¥ΖΫΖ®ΈΣΫβ¥πΒΡΙΊΦϋΘ§≤ύ÷ΊΗ¥Ζ÷ΫβΖ¥”ΠΦΑ―θΜ·ΜΙ‘≠Ζ¥”ΠΒΡάκΉ”Ζ¥”ΠΩΦ≤ιΘ§ΧβΡΩΡ―Ε»≤Μ¥σΘ°

ΝΖœΑ≤αœΒΝ–¥πΑΗ
 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
œύΙΊΧβΡΩ
4Θ°œ¬Ν– Β―ι≤ΌΉςΡή¥οΒΫ‘ΛΤΎΡΩΒΡ «Θ®ΓΓΓΓΘ©
| AΘ° | ≈δ÷Τ“χΑ±»ή“ΚΘΚœρœΓΒΡAgNO3»ή“Κ÷–÷πΒΈΦ”»κœΓΑ±Υ°÷ΝΗ’ΚΟ…ζ≥…≥ΝΒμ | |
| BΘ° | ≥ΐ»Ξ±Ϋ÷–ΒΡ…ΌΝΩ±ΫΖ”ΘΚΦ”»κ≈®δεΥ°»ΜΚσΙΐ¬Υ | |
| CΘ° | ≥ΐ»Ξ““Υα““θΞ÷–ΒΡ…ΌΝΩ““ΥαΘΚΦ”»κ±ΞΚΆΧΦΥαΡΤ»ή“ΚΘ§≥δΖ÷’ώΒ¥Θ§Ψ≤÷ΟΖ÷“ΚΘ§ΤζΥ°≤ψ | |
| DΘ° | Φλ―ιδε““Άι÷–ΒΡδεΘΚΫΪδε““Άι”κNaOH»ή“ΚΙ≤»»Κσά以ȧ‘ΌΒΈΦ”AgNO3»ή“Κ |
9Θ°‘ΎΟή±’»ίΤς÷–Ϋχ––œ¬Ν–Ζ¥”ΠΘΚMΘ®gΘ©+NΘ®gΘ©?RΘ®gΘ©+2L¥ΥΖ¥”ΠΖϊΚœ»γΆΦΘ§œ¬Ν––π ω’ΐ»ΖΒΡ «Θ®ΓΓΓΓΘ©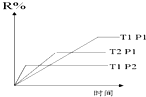

| AΘ° | ’ΐΖ¥”ΠΈϋ»»Θ§L «ΤχΧε | BΘ° | ’ΐΖ¥”ΠΈϋ»»Θ§L «ΙΧΧε | ||
| CΘ° | ’ΐΖ¥”ΠΖ≈»»Θ§L «ΤχΧε | DΘ° | ’ΐΖ¥”ΠΖ≈»»Θ§L «ΙΧΧε |
19Θ°Α¥Χ«άύΒΡœ÷¥ζΕ®“εΘ§œ¬Ν–Έο÷ ≤Μ τ”ΎΧ«άύΒΡ «Θ®ΓΓΓΓΘ©
ΔΌ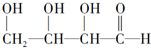 ΔΎ
ΔΎ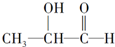
Δέ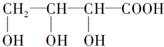 Δή
Δή
ΔΌ
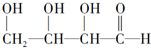 ΔΎ
ΔΎ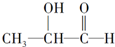
Δέ
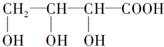 Δή
Δή
| AΘ° | ΔΎΔέ | BΘ° | ΔΌΔή | CΘ° | ΔέΔή | DΘ° | ΔΎΔή |
 Θ§
Θ§

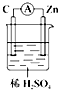
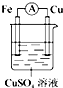
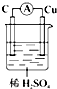
 Θ§Οϊ≥Τ «ΜΖΦΚΆιΘ°
Θ§Οϊ≥Τ «ΜΖΦΚΆιΘ°