��Ŀ����
����Ŀ����֪ͭ�������A�ṹ��ͼ����ش��������⣺

(1)д����̬Cu����Χ�����Ų�ʽ_____________________________________��
(2)���就�������(H2NCH2COO��)���ȷֽ�ɲ���CO2��N2��N2��������������Ŀ֮����__________��N2O��CO2��Ϊ�ȵ����壬��N2O�ĵ���ʽΪ____________��
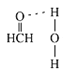
(3)��Cu���£��״��ɱ�����Ϊ��ȩ����ȩ������HCO�ļ���_____(ѡ������������������������С����)120������ȩ����ˮ�γ������������ͼ�б�ʾ����_____��

(4)������������ͼ����ʯ�ṹ���ƣ��dz�Ӳ���ϡ���������������B-N��������ԭ����֮��Ϊ___________��

(5)Cu����Ķѻ���ʽ��ͼ��ʾ����Cuԭ�Ӱ뾶Ϊa��������Cuԭ�ӵ���λ��Ϊ________������Ŀռ�������Ϊ______��(��֪:![]() ����ʽ����������)
����ʽ����������)

���𰸡�3d104s1 1��2 ![]() ����
����  4��1 12 74.76%
4��1 12 74.76%
��������
(1)Cuԭ�Ӻ�����29�����ӣ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽ��
(2)����N2�ĽṹʽΪN��N�ж�������������Ŀ֮�ȣ��ȵ�����Ľṹ���ƣ����ݶ�����̼����ʽ��дN2O�ĵ���ʽ��
(3)��ȩ��C��sp2�ӻ�����ƽ�������Σ���Ϲ¶Ե�����ɼ����ӶԵ����÷����жϼ��ǵĴ�С����ȩ�����е�Oԭ�Ӻ�ˮ�����е�Hԭ�����γ������
(4)�þ�����Nԭ���ھ����ڲ���ÿ��Nԭ���γ�4��B-N���������ж�B-N��������Bԭ�Ӹ���֮�ȣ�
(5)Cu����Ķѻ���ʽ�������������ܶѻ����ݴ��ж���λ�������������ĶԽ��߳���4r���������������ⳤ�;�������������ݾ����Ľṹ�����Cuԭ�ӵĸ����������ݾ���Ŀռ�������=![]() ��100%���㡣
��100%���㡣
(1)Cuԭ�Ӻ�����29�����ӣ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽΪ[Ar]3d104s1�����Ի�̬Cu����Χ�����Ų�ʽΪ��3d104s1���ʴ�Ϊ��3d104s1��
(2)N2�ĽṹʽΪN��N��������������Ŀ֮��Ϊ1��2���ȵ�����Ľṹ���ƣ����ݶ�����̼����ʽ������дN2O�ĵ���ʽΪ![]() ���ʴ�Ϊ��1��2��
���ʴ�Ϊ��1��2��![]() ��
��
(3)��ȩ��C��sp2�ӻ�����ƽ�������Σ�Cԭ�����������ڲ���C-H��C-H���н���������120�㣬���������ʻ����ŶԵ��ӵ��ų⣬ʵ�ʼ���Ӧ����С��120�㣬���Լ�ȩ������HCO�ļ��Ǵ���120�㣻��ȩ�����е�Oԭ�Ӻ�ˮ�����е�Hԭ�����γ�������������ʾΪ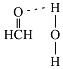 ���ʴ�Ϊ�����ڣ�
���ʴ�Ϊ�����ڣ� ��
��
(4)�þ�����Nԭ���ھ����ڲ���������4��ÿ��Nԭ���γ�4��B-N����B-N������Ϊ16��Bԭ��λ�ھ�����������ģ�����=8��![]() +6��
+6��![]() =4����B-N����Bԭ�Ӹ���֮��Ϊ16��4=4��1���ʴ�Ϊ��4��1��
=4����B-N����Bԭ�Ӹ���֮��Ϊ16��4=4��1���ʴ�Ϊ��4��1��
(5)Cu����Ķѻ���ʽ�������������ܶѻ���������λ����12����ͼ��֪�����������ĶԽ��߳���4a��������������ⳤ=2![]() a��1��Cu�������
a��1��Cu�������![]() ��a3��������Cuԭ�ӵĸ���=8��
��a3��������Cuԭ�ӵĸ���=8��![]() +6��
+6��![]() =4�������Ծ���Ŀռ�������Ϊ
=4�������Ծ���Ŀռ�������Ϊ![]() ��100%=74.76%���ʴ�Ϊ��12��74.76%��
��100%=74.76%���ʴ�Ϊ��12��74.76%��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���о���ѧ��Ӧʱ����Ҫ�������ʱ仯�������仯����Ҫ��ע��Ӧ�Ŀ������ȡ��ش���������:
(1)NH3��ԭNO����Ҫ�����������������䷴Ӧ������������ϵ��ͼ��ʾ

����ͼ����Ϊ�ı��˷�Ӧ��������Ӧ�Ļ�ܣ�b_______(����������������������)a��
��������Ӧ���Ȼ�ѧ����ʽ�ɱ�ʾΪ��Ӧ�����������H��______(��E1��E2�Ĵ���ʽ��ʾ)��
���о����֣�һ�������µ�������Ӧ���̿�����ͼ��ʾ������������ԭ��Ӧ�����ʵ����ã�NOΪ_______���������ܷ�Ӧ�Ļ�ѧ����ʽΪ_______________��

(2)һ���¶��£�����ͬ���ʵ�����H2O(g)��CO�ֱ�ͨ���ݻ�Ϊ1L�ĺ����������У����з�ӦH2O(g)��CO(g)![]() CO2(g)��H2(g)���õ������ʾ����������
CO2(g)��H2(g)���õ������ʾ����������
������ | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ��ʱ��/min | ||
H2O(g) | CO(g) | CO(g) | H2(g) | |||
1 | 650 | 2.0 | 4.0 | 3.0 | 1.0 | 5 |
2 | 900 | 1.0 | 2.0 | 1.8 | 0.2 | 4 |
3 | 900 | a | b | c | d | t |
��4mim�ڣ�ʵ��2��v(CO2)��______�� 900��ʱ����Ӧ��ƽ�ⳣ��Ϊ______�������¶�ʱ��ƽ�ⳣ����________(��������������С������������)��
��650��ʱ�����ڴ������г���2.0 mol H2O(g)��1.0molCO(g)��1.0 mol CO2(g)�� xmol H2(g)��Ҫʹ��Ӧ�ڿ�ʼʱ������Ӧ������У���xӦ�����������__________��
��a��2.0��b��1.0����ƽ��ʱʵ��2��H2O(g)��ʵ��3��CO(g)��ת����(a)�Ĺ�ϵΪa(H2O) _______ (�������������ɣ���)a(CO)��





