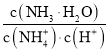��Ŀ����
����Ŀ����(N2H4)��Ϊ�����������ȼ�ϣ���ͨ����ӦNaClO + 2NH3 = N2H4 + NaCl + H2O��ȡ��ij��ѧ��ȤС�鳢����ʵ������ȡN2H4�����������ʵ�飺
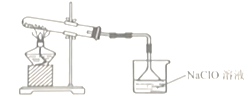
(1)�Ʊ�NaClO��Һ��װ����ͼ��ʾ��(��֪��3Cl2 + 6NaOH![]() 5NaCl + NaClO3 + 3H2O)
5NaCl + NaClO3 + 3H2O)

������A��������________________��
�����Ӻ�װ�ã�װҩƷ֮ǰ��������е�һ�������_________��
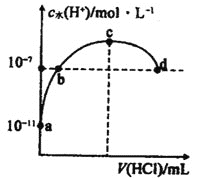
��Բ����ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽΪ_______________��������71g����ʱ����������HClΪ_________mol���Թ��ڷ�����Ӧ�����ӷ���ʽΪ_________��
�ܱ���ʳ��ˮ��������_____________����ˮ��������____________��
(2)��NaClO��Һ�����ձ��У�����ͨ��NH3��ȡN2H4����ȡ�����Ļ�ѧ����ʽΪ_______��
(3)�������ʱ��N2H4��ȼ�ϣ���N2O4��ȼ��ȼ���������ֿɲ������ѭ�������ʡ�д���÷�Ӧ�Ļ�ѧ����ʽ______________��
���𰸡���Һ©�� ���װ�õ������� MnO2 + 4HCl(Ũ)![]() MnCl2 + Cl2�� + 2H2O 2 Cl2 + 2OH�� = Cl�� + ClO�� + H2O ��ȥCl2�е�HCl���� ��ֹ��Һ�¶ȹ��߷�������Ӧ 2NH4Cl + Ca(OH)2
MnCl2 + Cl2�� + 2H2O 2 Cl2 + 2OH�� = Cl�� + ClO�� + H2O ��ȥCl2�е�HCl���� ��ֹ��Һ�¶ȹ��߷�������Ӧ 2NH4Cl + Ca(OH)2![]() CaCl2 + 2NH3�� + 2H2O 2N2H4 + N2O4
CaCl2 + 2NH3�� + 2H2O 2N2H4 + N2O4![]() 3N2 + 4H2O
3N2 + 4H2O
��������
(1) �ٸ��������Ĺ����жϣ�
�ڼ��װ�õ������ԣ��Է�ֹ©����
�۸�װ���������Ʊ������ģ�MnO2 ��Ũ���ᷴӦ����MnCl2 �� Cl2��H2O���Թ�������������������Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ��
�ܱ���ʳ��ˮ�������dz�ȥCl2�е�HCl���壬��ˮ�������Ƿ�ֹ��Һ�¶ȹ��߷�������Ӧ��
(2) ʵ�������ü����Ȼ�狀��������ƻ�Ϲ�����ȡ������
(3)�������ʱ��N2H4��ȼ�ϣ���N2O4��ȼ��ȼ���������ֿɲ������ѭ�������ʵ�����ˮ������
(1) �ٸ��������Ĺ����֪������A�������Ƿ�Һ©����
�ڻ�ѧʵ��װ�������Ժ���װҩƷ֮ǰ��������е�һ������Ǽ��װ�õ������ԣ��Է�ֹ©����
�۸�װ���������Ʊ������ģ�����Բ����ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽΪMnO2 + 4HCl(Ũ)![]() MnCl2 + Cl2�� + 2H2O�����ݷ�Ӧ��֪��������71g����ʱ����������HClΪ2mol���Թ�������������������Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ��������Ӧ�����ӷ���ʽΪCl2 + 2OH�� = Cl�� + ClO�� + H2O��
MnCl2 + Cl2�� + 2H2O�����ݷ�Ӧ��֪��������71g����ʱ����������HClΪ2mol���Թ�������������������Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ��������Ӧ�����ӷ���ʽΪCl2 + 2OH�� = Cl�� + ClO�� + H2O��
�ܱ���ʳ��ˮ�������dz�ȥCl2�е�HCl���壬��ˮ�������Ƿ�ֹ��Һ�¶ȹ��߷�������Ӧ��
(2) ʵ�������ü����Ȼ�狀��������ƻ�Ϲ�����ȡ��������Ӧ�Ļ�ѧ����ʽΪ2NH4Cl + Ca(OH)2![]() CaCl2 + 2NH3�� + 2H2O��
CaCl2 + 2NH3�� + 2H2O��
(3)�������ʱ��N2H4��ȼ�ϣ���N2O4��ȼ��ȼ���������ֿɲ������ѭ�������ʵ�����ˮ��������Ӧ�Ļ�ѧ����ʽΪ2N2H4 + N2O4![]() 3N2 + 4H2O��
3N2 + 4H2O��

 ��У����ϵ�д�
��У����ϵ�д�