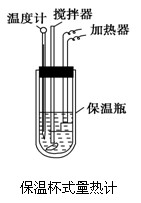
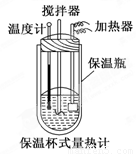
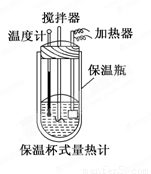
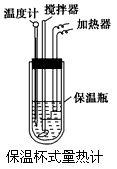
��Ŀ����
�����ȼ���(��ͼ)��100 mL 0.50 mol��L-1��CH3COOH��Һ��100 mL 0.55 mol��L-1��NaOH��Һ��ϣ��¶ȴ�298.0 K������300.7 K����֪���ȼƵ����ݳ���(���ȼƸ�����ÿ����1 K����Ҫ������)��150.5 J��K-1����Һ�ܶȾ�Ϊ1 g��mL-1��������Һ�ı�����c��4.184 J��(g��K)-1��
(1)����CH3COOH���к��Ȧ�H��_____________________
(2)CH3COOH���к��ȵ�����ֵΪ56.1 kJ��mol-1������Ϊ(1)�в�õ�ʵ��ֵ��ƫ����ܵ�ԭ����____________________________________��
(3)ʵ����NaOH������Ŀ����___________________________________________��
(4)����ΪCH3COOH���к�����HCl���к�����ֵ��ȣ�________�ϴ���ԭ����_______________��
(2)CH3COOH���к��ȵ�����ֵΪ56.1 kJ��mol-1������Ϊ(1)�в�õ�ʵ��ֵ��ƫ����ܵ�ԭ����____________________________________��
(3)ʵ����NaOH������Ŀ����___________________________________________��
(4)����ΪCH3COOH���к�����HCl���к�����ֵ��ȣ�________�ϴ���ԭ����_______________��
(1)��H��-53.3 kJ��mol-1��
(2)�����ȼƵı���ƿ����Ч�����ã��������Һ��ϲ�Ѹ�٣����¶ȼƲ�����ȷ�ȡ�
(3)�����ļ��ܱ�֤CH3COOH��ȫ���кͣ��Ӷ����ʵ��ȷ��
(4)HCl��CH3COOH�����ᣬֻ���ٲ��ֵ��룬CH3COOH��������ʱҪ���ȣ��к�ʱ���Ƚ���
(2)�����ȼƵı���ƿ����Ч�����ã��������Һ��ϲ�Ѹ�٣����¶ȼƲ�����ȷ�ȡ�
(3)�����ļ��ܱ�֤CH3COOH��ȫ���кͣ��Ӷ����ʵ��ȷ��
(4)HCl��CH3COOH�����ᣬֻ���ٲ��ֵ��룬CH3COOH��������ʱҪ���ȣ��к�ʱ���Ƚ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ