��Ŀ����
�ס��ҡ�������4�����ʷֱ�2�ֻ�3��Ԫ�أ����ǵķ����и���18�����ӡ�������̬�⻯���ˮ�зֲ���������������ӡ������ƶϺ�������
A��ij������Һ��������������ӣ������Һ�Լ��ԣ�ֻ�����ᷴӦ
B������������Ħ��������ͬ������һ�����м��Լ��ͷǼ��Լ�
C�����к���2����IVA���Ԫ�أ����һ���Ǽ����ͬϵ��
D�����ͼ��и�Ԫ����������ͬ������һ������-1�۵�Ԫ��
D
����:���⿼�����ʽṹ֪ʶ��ѡ��A������18���ӵ��⻯�����ˮ��ҺΪ��Ԫ���ᣬ���ѵó���ΪH2S������NaHS��Һ�к���HS�D��S2�D����NaHS��������ȷ�Ӧ����H2S��ѡ��B��O2��Ħ������Ϊ32g/mol���ҵ�Ħ������ҲΪ32g/mol���Һ���18���ӣ���CH3OH���ϣ�CH3OH��ֻ���м��Լ��Ǽ��Լ���ѡ��C����2���ڢ�A��Ԫ��ΪC����CH3OH���ϣ���CH3OH����CH4��ͬϵ�ѡ��D��H2S��Ԫ�ص�������Ϊ1/16(H/S)��H2O2������Ԫ�ص�������ҲΪ1/16(H/O)��H2O2����Ԫ�صļ�̬Ϊ��1�ۣ����ϡ�

 ��У����ϵ�д�
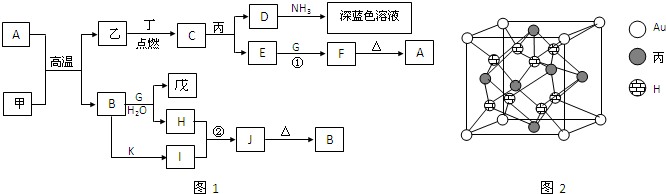
��У����ϵ�д���9�֣�A��B��C��D��E���ֶ�����Ԫ�أ���֪�����ǵ�ԭ��������������A��B����Ԫ�صĺ˵����֮��������ǵ�ԭ������������֮�ͣ�Bԭ���������������������������2����Ԫ�����ڱ��У�C��E�IJ�ͬ��������Ԫ�أ�D��E��ԭ������֮��Ϊ30�����������γɵĻ�����Ϊ�ס��ҡ����������֡������ֻ�������ԭ�Ӹ��������±�������Ԫ�ط�������
| | �� | �� | �� | �� |
| �������и�Ԫ��ԭ�Ӹ����� | A��C=1��1 | B��A=1��2 | D��E=1��3 | B��E=1��4 |
A�� B�� C�� D�� E��
��2�����ˮ��Һ�м���MnO2������������ ��
��3����֪�л����ҵķ���Ϊƽ��ṹ��̼�������Ϊ120�㣬ʵ������ȡ�ҵĻ�ѧ����ʽΪ��
��4������ˮ��Һ�����ԣ��뱥��NaHCO3��Һ��Ӧ�����������
 ���������й����ӷ���ʽ�ǣ�
���������й����ӷ���ʽ�ǣ�