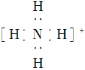��Ŀ����
����Ŀ������Ԫ��Fe��Ni��Pt��Ϊ���ڱ���ͬ��Ԫ�أ�����Ԫ�صĻ��������о�����������������Ҫ��;��
(1)Fe�dz����Ľ�������������;�㷺����ش��������⣺
��Fe��Ԫ�����ڱ��е�λ��Ϊ________________________��
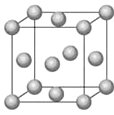
��Fe��һ�־����ṹ��ͼ��ʾ����þ����߳�Ϊ a pm����Feԭ�Ӱ뾶Ϊ__________��
����֪��FeO���徧���ṹΪNaCl�ͣ���O2����������ҵȾ������������Χ�ɵĿռ乹��Ϊ________��
(2)�����벻ͬ�������γɶ�����������ʽΪ[Pt(NH3)2Cl4]��������������_________��������������ֲ�ͬ����ɫ�����гȻ�ɫ�Ƚϲ��ȶ�����ˮ�е��ܽ�ȴ�����ɫ��������ˮ�е��ܽ��С������ͼ��ʾ�Ľṹʾ��ͼ�г�����ɫ����_____(����A������B��)��������___________��
A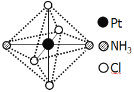 B
B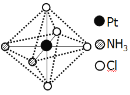
(3)����������(La)�γɵĺϽ���һ�����õĴ�����ϣ��侧���ṹʾ��ͼ��ͼ��
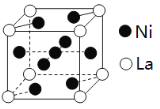
�ٲ�֪�����Ͻ����Ϊ9.0��10��23cm3���������Ͻ�ľ����ܶ�Ϊ____________ (���ؼ�����) ��
�ڴ���ԭ��Ϊ�������Ͻ�����H2��H2����Ϊԭ�ӣ�Hԭ�Ӵ����������γɻ�������������ԭ��ռ�ݾ��������µ����������ģ����γɵĴ��⻯����Ļ�ѧʽΪ____________��
(4)��֪����ԭ�ӷ����У���ԭ�Ӷ���ͬһƽ��������Щԭ�����ƽ�е�p�������p���ӿ��ڶ��ԭ�Ӽ��˶����γ�������������(�������)������������![]() ��ʾ������m��n�ֱ���������γɴ������ĵ�������ԭ�Ӹ������籽�����д�������ʾΪ
��ʾ������m��n�ֱ���������γɴ������ĵ�������ԭ�Ӹ������籽�����д�������ʾΪ![]() �� �������д�������������������__________��
�� �������������������������__________��
a.O3 b.SO42- c.H2S d.NO3-
���𰸡���4���ڵڢ��� ![]() pm ���������� NH3��Cl�� A B��ˮ��Ϊ���Է��ӣ��������ܣ�AΪ�Ǽ��Է�����ˮ�е��ܽ��С
pm ���������� NH3��Cl�� A B��ˮ��Ϊ���Է��ӣ��������ܣ�AΪ�Ǽ��Է�����ˮ�е��ܽ��С ![]() gcm-3 LaNi5H3 ad
gcm-3 LaNi5H3 ad
��������
(1)������26��Ԫ�أ��ݴ��ж������ڱ��е�λ�ã��ڸ��ݾ����ṹ֪����Խ���Ϊ��ԭ�Ӱ뾶��4�����ݴ˼�����ԭ�Ӱ뾶����FeO���徧���ṹΪNaCl�ͣ���O2-��������ҵȾ������������Ϊ������������ϵ����ӣ��ݴ��жϣ�
(2)�ؼ�ͼʾ�ж�Pt(NH3)2Cl4�����������壻������������ԭ�������жϣ�
(3)�ٸ��ݾ����ṹͼ�����þ�̯������La��Ni�ĸ���������ܶ�=![]() ���㣻�ڸ�����ԭ��ռ�ݾ��������µ����������ģ��������þ�̯��H�ĸ�������Ϣ��жϻ�ѧʽ��
���㣻�ڸ�����ԭ��ռ�ݾ��������µ����������ģ��������þ�̯��H�ĸ�������Ϣ��жϻ�ѧʽ��
(4)�����γ������������γ���������ԭ�Ӷ���ͬһƽ��������Щԭ�����ƽ�е�p����������������жϡ�
(1)������26��Ԫ�أ�λ�ڵ������ڵ�VIII�壬�ʴ�Ϊ���������ڵ�VIII�壻
�ڸ��ݾ����ṹ֪����Խ���Ϊ��ԭ�Ӱ뾶��4������Խ��߳���=![]() a pm����Feԭ�Ӱ뾶=
a pm����Feԭ�Ӱ뾶=![]() pm=
pm=![]() pm���ʴ�Ϊ��
pm���ʴ�Ϊ��![]() pm��
pm��
��FeO���徧���ṹΪNaCl�ͣ���O2-��������ҵȾ������������Ϊ������������ϵ����ӣ���O2-�Ⱦ����������Fe2+Χ�ɵĿռ乹��Ϊ�������壬�ʴ�Ϊ���������壻
(2)���ݽṹͼ��[Pt(NH3)2Cl4]������ΪNH3��Cl-���Ȼ�ɫ��ˮ�е��ܽ�Ƚϴ�ˮΪ���Է��ӣ������������ܣ��Ȼ�ɫҲΪ���Է��ӣ�AΪ�Գƽṹ�����ڷǼ��Է��ӣ�BΪ�ǶԳƽṹ�����ڼ��Է��ӣ���Ȼ�ɫ�������ΪB������ɫ��ΪA���ʴ�Ϊ��NH3��Cl-��A��B��ˮ��Ϊ���Է��ӣ��������ܣ�AΪ�Ǽ��Է�����ˮ�е��ܽ��С��
(3)�ٸ��ݾ����ṹͼ��La�ĸ���Ϊ8��![]() =1��Ni�ĸ���Ϊ8��
=1��Ni�ĸ���Ϊ8��![]() +1=5������������Ϊ
+1=5������������Ϊ![]() g���ܶ�=
g���ܶ�=![]() =
=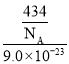 gcm-3=
gcm-3=![]() gcm-3���ʴ�Ϊ��
gcm-3���ʴ�Ϊ��![]() gcm-3��
gcm-3��
�ڸ��ݾ����ṹͼ��La�ĸ���Ϊ8��![]() =1��Ni�ĸ���Ϊ8��
=1��Ni�ĸ���Ϊ8��![]() +1=5����ԭ��ռ�ݾ��������µ����������ģ���H�ĸ���Ϊ8��
+1=5����ԭ��ռ�ݾ��������µ����������ģ���H�ĸ���Ϊ8��![]() +2��
+2��![]() =3���仯ѧʽΪLaNi5H3���ʴ�Ϊ��LaNi5H3��
=3���仯ѧʽΪLaNi5H3���ʴ�Ϊ��LaNi5H3��
(4)�γ������������γ���������ԭ�Ӷ���ͬһƽ��������Щԭ�����ƽ�е�p����� ��O3ΪV�νṹ�����ƽ�е�p����������γ������������������������������ṹ��ԭ�Ӳ�����ͬһƽ���ڣ������γ�����������������Hԭ�Ӻ�Sԭ��û��ƽ�е�p����������γ�����������NO3-Ϊƽ�������Σ����ƽ�е�p����������γ������������ʴ�Ϊ��ad��

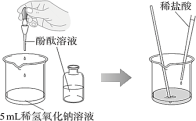
����Ŀ��ijС��ͬѧ���ʵ�飬̽���кͷ�Ӧ�ķ�����
��ʵ����̣�
��� | ʵ����� |
ʵ��I | ����з�̪������������Һ�еμ�ϡ���ᣬ�۲�����
|
ʵ��II | ����ͼ����ʵ�飬�ԱȢ١����е�ʵ������
|
����������ͣ�
(1)�кͷ�Ӧ��ʵ����______(�����ӷ���ʽ��ʾ)��
(2)ʵ��I�У��кͷ�Ӧ������������______��
(3)ʵ��II�У����ж��кͷ�Ӧ������������______��
a.��Ӧ��������þ�������ڣ���
b.þ������������ݵ����ʢ٣���
(4)ʵ��II�У�����ṩ�¶ȼơ�����(�ռ�����)�ȸ������������ܻ�ȡ֤���кͷ�Ӧ������ʵ��֤����______��