题目内容
【题目】铁元素和碳元素形成的单质及化合物在生产、生活中有广泛的用途,
请回答下列问题:
(1)基态Fe原子的价层电子的电子排布图为_________________;其最外层电子的电子云形状为___________。
(2)(NH4)2Fe(SO4)2 6H2O俗称摩尔盐。其阴离子的VSEPR模型名称为____________________。
写出一种与NH4+互为等电子体的分子的电子式:________________________________。
(3)Fe(CO)5可用作催化剂、汽油抗暴剂等.其分子中σ键和π键的数目之比为______________。CO的沸点高于N2的原因是_________________________。
(4)碳元素可形成多种单质。
①石墨烯是从石墨中剥离出来的由单层碳原子构成的平面结构新型碳材料。其中碳原子的杂化方式为______________________。
料,其中碳原子的杂化方式为 ,
②金刚石的晶胞如图所示。若晶胞参数为a pm,阿伏加德罗常数的值为NA,则该晶胞中原子的体积占晶胞体积的百分率为________________;1cm3晶体的平均质量为___________(列出计算式即可)。
【答案】  球形 正四面体形
球形 正四面体形 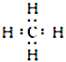 (或
(或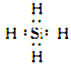 ) 1∶1 二者相对分子质量相同,CO为极性分子,N2为非极性分子,CO的分子间作用力大于N2的 sp2 34%
) 1∶1 二者相对分子质量相同,CO为极性分子,N2为非极性分子,CO的分子间作用力大于N2的 sp2 34% ![]()
【解析】(1)基态 F e原子的价层电子排布式为3d64s2,可得其价层电子的电子排布图。 其最外层电子为 4s电子,故电子云形状为球形。
(2)SO42- 的价层电子对数目为4,其 VSEPR 模型名称为正四面体形。 根据等电子体的含义:原子总数相同且价电子数相等,与 NH4 + 互为等电子体的分子为CH4 或SiH4,可写出电子式。(3)Fe(CO)5分子中 Fe原子与5个 CO 形成5个σ键,每个CO 分子中含有1个σ键和2个π键,故二者的数
目之比为1∶1。 二者相对分子质量相同,组成和结构相似,极性越强,分子间作用力越大,沸点越高。
(4)①石墨中碳原子与其他三个碳原子之间形成三个σ键,其空间构型为平面三角形, 故杂化 方式为sp2。
②空间利用率等于晶胞中原子实际占用体积除以晶胞体积,可得其空间利用率为34%。每个金刚石的晶胞实际占用8个碳原子,其质量为(12×8)/ NA g;晶胞的体积为( a×10-10)3cm3,1cm3 晶体中平均含有晶胞的数目为1/(a×10-10)3,则1cm3 晶体的平均质量为![]() 。
。

【题目】等质量的C2H4和NH3相比较,下列结论错误的是
A. 它们的分子个数比为17:28
B. 它们的原子个数比为17:28
C. 它们的氢原子个数比为17:21
D. 它们在相同状况下的体积比17:21