��Ŀ����
��6�֣���֪����1mol H2�еĹ��ۼ���������436KJ������0.5mol O2�еĹ��ۼ���������249KJ�������γ�1molˮ�����еĹ��ۼ��ͷ�930KJ ������
��H2O(l)��H2O(g) ��H��+44 kJ��mol-1
��CH4 ( g ) + 2O2 ( g ) = 2H2O ( l ) + CO2 ( g ) ��H = ��890 kJ��mol-1
��1��д��H2 ��O2��ȼ������ˮ�������Ȼ�ѧ����ʽ ��
��2��1.12L������ɱ���µ������H2��ȫȼ������Һ̬ˮ�ų�������Ϊ ��
��3��ʵ���������ͼ���Ļ�����干4 mol��ȫȼ��ʱ����Ϊ2358kJ�����ɵ�ˮΪҺ̬�������������������ͼ���������Ϊ ��
��ÿС��2�֣���1��H2(g)+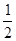 O2(g)==H2O(g) ��H= ��245kJ/mol
O2(g)==H2O(g) ��H= ��245kJ/mol
��2��14.45KJ ��3��1:1
�����������鷴Ӧ�ȷ��ȼ��㼰�Ȼ�ѧ����ʽ����д
��1����Ӧ�Ⱦ��Ƕϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ����˷�Ӧ����436KJ/mol+249KJ/mol��930KJ/mol��245kJ/mol�����Է�Ӧ���Ȼ�ѧ����ʽ��H2(g)+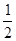 O2(g)==H2O(g) ��H= ��245kJ/mol��
O2(g)==H2O(g) ��H= ��245kJ/mol��
��2����״����1.12L������0.05mol��ȼ������ˮ����������245kJ/mol��0.05mol��12.25kJ������ˮ������0.05mol�����Һ̬ˮ�ַų�������44kJ/mol��0.05mol��2.2kJ���������շų���������12.25kJ��2.2kJ��14.45KJ��
��3�����������������ͼ�������ʵ����ֱ���x��y����x+y��4mol�������Ȼ�ѧ����ʽ��֪��289kJ/mol��x+890 kJ/mol��y��2358kJ�����x��y��2mol�����Զ��ߵ����֮����1:1��

 2NH3�У�ÿ����2mol NH3��
2NH3�У�ÿ����2mol NH3��