��Ŀ����
����Ŀ����ͼA��E����ѧ��ѧ���õ�����������
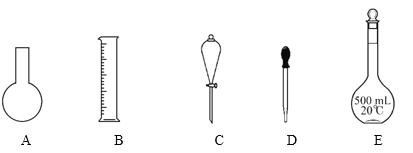
�ش��������⣺
(1)���������У�ʹ��ʱ�����ȼ���Ƿ�©ˮ����_________�����ţ���
(2)����ʵ������У�ʹ�õ�����C����________�����ţ���
a������ˮ�;ƾ��Ļ����
b������ˮ�����Ȼ�̼�Ļ����
c������ˮ����ɰ�Ļ����
d���ᴿFe(OH)3����
(3)ij��ѧ��ȤС��ʵ������У���Ҫ480 mL0.5mol��L��1 NaOH��Һ��
�ٳ������ṩ�������⣬���õ��IJ���������_________________��
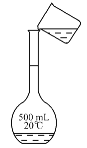
����ͼ��ʾ��ת�Ʋ����е�һ��������_____________________��
�������ƹ����У���������ᵼ��������ҺŨ��ƫ�͵���_______�����ţ���
a��ȷ����9.6gNaOH
b���ܽ�ʱδ��ȴ�����¾�ת��
c���ڶ���ʱ�����ӿ̶���
d����ˮ���̶��ߺ�ҡ�ȣ�����Һ����ڿ̶���
���𰸡�CE b �ձ��������� ת����Һʱδ�ò��������� ac
��������
(1)�л��������ӵ�������ʹ��ǰ�����©��
(2)����CΪ��Һ©�������ڷ��뻥�����ܵ�Һ�����
(3)������480 mL0.5mol��L��1 NaOH��Һ����Ҫ�������У�500mL����ƿ����ƽ(����Ͳ)���ձ�������������ͷ�ιܣ�
��ת�Ʋ�����Ҫʹ�ò�����������
�۸���c=![]() �����жϡ�
�����жϡ�
(1)AΪԲ����ƿ��BΪ��Ͳ��CΪ��Һ©����DΪ��ͷ�ιܣ�EΪ500mL����ƿ���л��������ӵ�������ʹ��ǰ�����©��������װ����ֻ�з�Һ©����500mL����ƿ�����ӣ���ѡCE��
(2)����CΪ��Һ©�������ڷ��뻥�������ҷֲ��Һ�����
a��ˮ�;ƾ�����Ȼ��ܣ������÷�Һ©�����룬��a���������⣻
b��ˮ�����Ȼ�̼�������ҷֲ㣬�����÷�Һ©�����룬��b�������⣻
c��ˮ����ɰ�����ܣ�����ɳ����Һ�壬һ��ʹ�ù��˵ķ������룬��c���������⣻
d�� Fe(OH)3������ˮ��ϲ��ֲ㣬�����÷�Һ©�����룬һ���������ķ������룬��d���������⣻
��ѡb��
(3) ������480 mL0.5mol��L��1 NaOH��Һ����Ҫ�������У� 500mL����ƿ����ƽ(����Ͳ)���ձ�������������ͷ�ιܣ��������ṩ�������⣬���õ��IJ����������ձ�����������
��ת�Ʋ�����Ҫʹ�ò������������ʲ����е�һ��������ת����Һʱδ�ò�����������
��a������480 mL0.5mol��L��1 NaOH��Һ��Ҫʹ��500mL����ƿ����Ҫ���ʵ�����=0.5L��0.5mol/L��40g/mol=10g������9.6gNaOH�����ʵ����ʵ���ƫ�ͣ���������Һ��Ũ��ƫ�ͣ���a�������⣻
b���ܽ�ʱδ��ȴ�����¾�ת�ƣ���ȴ����Һ��������С����������Һ��Ũ��ƫ�ߣ���b���������⣻
c���ڶ���ʱ�����ӿ̶��ߣ���Һ�����ƫ����������Һ��Ũ��ƫ�ͣ���c�������⣻
d����ˮ���̶��ߺ�ҡ�ȣ�����Һ����ڿ̶��ߣ����ú�Һ���ָ����̶��ߣ���������ҺŨ����Ӱ�죬��d���������⣻
��ѡac��

����Ŀ������ʵ���о��к���ɫ����������Աȷ������ý��۲���ȷ���ǣ� ��
|
|
|
�� | �� | �� |
A.�ɢ��еĺ���ɫ���壬�ƶϲ���������һ���ǻ������
B.����ɫ���岻�ܱ�������ľ̿��Ũ���ᷢ���˷�Ӧ
C.�ɢ�˵��Ũ������лӷ��ԣ����ɵĺ���ɫ����Ϊ��ԭ����
D.�۵���������м���CO2���ɴ�˵��ľ̿һ����Ũ���ᷢ���˷�Ӧ










