��Ŀ����
�������ӷ���ʽ����д����۾���������
| ѡ�� | ���ӷ���ʽ | ���� |
| A | AgCl(s) + I-( aq)  AgI(s) + Cl-(aq) AgI(s) + Cl-(aq) | �ܽ�ȣ�AgI >AgCl |
| B | Fe2��+ H2O2 +2H��= Fe3��+2H2O | �����ԣ�H2O2 > Fe3�� |
| C | CO32- + CO2 + H2O = 2HCO3- | �ȶ��ԣ�HCO3- >CO32- |
| D | NH3 + H3O+ = NH4+ + H2O | ������������NH3>H2O |
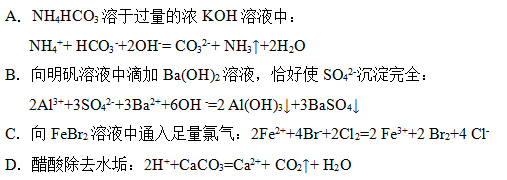
D
�������������A.AgCl����Һ�д��ڳ����ܽ�ƽ�⣺AgCl(s) +  Ag+(aq) + Cl-(aq)������Һ�м���KI��Һʱ������c(Ag+)��c(I-)>Ksp(AgI),���ᷢ��������ӦAg++ I-= AgI���������ƻ���AgCl�ij����ܽ�ƽ�⣬AgCl�ͻ���ܽ⣬���ճ�����ΪAgI���ܷ���ʽΪAgCl(s) + I-( aq)
Ag+(aq) + Cl-(aq)������Һ�м���KI��Һʱ������c(Ag+)��c(I-)>Ksp(AgI),���ᷢ��������ӦAg++ I-= AgI���������ƻ���AgCl�ij����ܽ�ƽ�⣬AgCl�ͻ���ܽ⣬���ճ�����ΪAgI���ܷ���ʽΪAgCl(s) + I-( aq) AgI(s) + Cl-(aq)����֤�����ܽ��AgCl >AgI�������ܵ�������ܵ�ת��������B.���ӷ���ʽδ��ƽ��Ӧ��Ϊ2Fe2��+ H2O2 +2H��= 2Fe3��+2H2O.��������ԭ��Ӧ�������ԣ���������ǿ����������������ԡ������ԣ�H2O2 > Fe3��������D������NH3������ӵ�����ǿ����������Һ�з�����ӦNH3 + H3O+=NH4+ + H2O��ˮ�е����Ӷ�ȡ��������˿���֤��������������NH3>H2O����ȷ��
AgI(s) + Cl-(aq)����֤�����ܽ��AgCl >AgI�������ܵ�������ܵ�ת��������B.���ӷ���ʽδ��ƽ��Ӧ��Ϊ2Fe2��+ H2O2 +2H��= 2Fe3��+2H2O.��������ԭ��Ӧ�������ԣ���������ǿ����������������ԡ������ԣ�H2O2 > Fe3��������D������NH3������ӵ�����ǿ����������Һ�з�����ӦNH3 + H3O+=NH4+ + H2O��ˮ�е����Ӷ�ȡ��������˿���֤��������������NH3>H2O����ȷ��
���㣺�������ӷ���ʽ����д��Ӧ�õ�֪ʶ��

���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ����
A������ͨ��ˮ�У�Cl2 + H2O  2H+ + Cl��+ClO�� 2H+ + Cl��+ClO�� |
| B���������������м���HI��Һ��Fe(OH)3 + 3H+ = Fe3+ + 3H2O |
| C��NaAlO2��Һ��ͨ�����CO2��2AlO2��+ CO2 + 3H2O = 2Al(OH)3��+ CO32�� |
| D����ϡ�����ȥ�Թ��ڱ�����3Ag+ 4H+ + NO3�� = 3Ag+ + NO�� +2H2O |
�������ӷ���ʽ��ȷ����
| A��NO2ͨ��AgNO3��Һ�У�3NO2��H2O��2NO3����NO��2H�� |
| B��������SO2ͨ��NaOH��Һ�У�SO2��2OH��=SO32-��H2O |
| C������NaOH����NH4HCO3ϡ��Һ�У�NH4+��OH��=NH3��H2O |
| D��ͭƬ����ϡHNO3�У�Cu��2NO3����4H��=Cu2����2NO2����2H2O |
ij��Һ�к���NO3-��S2-��AlO2-��SO32-���������ӣ��������м�����������ᣬ�Ȳ����裬�ټ��������NaOH��Һ������Һ�����ʵ��������������������
| A��NO3- | B��S2- | C��AlO2- | D��SO32- |
����������ʵ�Ļ�ѧ������ȷ����
A����NH3ͨ����з�̪��ˮ�У���Һ��죺NH3+ H2O  NH3��H2O NH3��H2O   + OH�� + OH�� |
B��ͭ��Ũ���Ṳ�Ȳ������壺Cu��H2SO4(Ũ�� Cu2+ + SO42- �� H2�� Cu2+ + SO42- �� H2�� |
| C��CH3COONa��ˮ��Һ�ʼ��ԣ�CH3COO- + H2O �� CH3COOH + OH- |
| D����NaOH��Һ����Cl2��Cl2��2OH- �� 2Cl- + H2O |
ˮ��Һ���ܴ��������һ��������
| A��Na+��Al3+��Cl����CO32�� | B��H+��Na+��Fe2+��MnO4�� |
| C��K+��Ca2+��Cl����NO3�� | D��K+��NH4+��OH����SO42�� |
���л�ѧ��Ӧ�����ӷ���ʽ��ȷ���ǣ� ��
| A����ϡ��ˮ��ͨ�����CO2��NH3��H2O��CO2=NH4+��HCO3- |
| B������SO2ͨ��Ca(ClO)2��Һ�У�SO2��H2O��Ca2����2ClO��=CaSO3����2HClO |
| C����ϡ�����ܽ�FeS���壺FeS��2H��=Fe2����H2S�� |
| D������������Һ������ʵ�����ϡ�����ϣ�Ca2����OH����H����SO42-=CaSO4����H2O |
���и�����������Һ���ܹ��������棬����Һ��c(H��)��10��1 mol��L��1ʱ�����������������Һ��c(H��)��10��13mol��L��1ʱ�������ɳ�������������ӿ�����
A��Na����Ba2���� �� �� |
B��Ba2����K����Cl���� |
C��Mg2���� �� �� ��Cl�� ��Cl�� |
D��Fe2����Na���� �� �� |