��Ŀ����
һ���¶��£����ݻ�ΪV L���ܱ������н��з�Ӧ��aN(g)![]() bM(g)��M��N�����ʵ�����ʱ��ı仯������ͼ��ʾ��
bM(g)��M��N�����ʵ�����ʱ��ı仯������ͼ��ʾ��
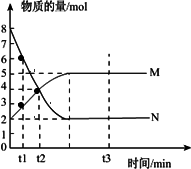
(1)�˷�Ӧ�Ļ�ѧ����ʽ��![]() ��________
��________
(2)t1��t2ʱ�̣���M��Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ��________
(3)������������˵��������Ӧ�ﵽƽ��״̬����________
A����Ӧ��M��N�����ʵ���֮��Ϊ1��1
B��������������������ʱ��ı仯���仯
C���������������ʵ�������ʱ��ı仯���仯
D����λʱ����ÿ����a mol��N��ͬʱ����b mol��M
E����������ѹǿ����ʱ��ı仯���仯
F��N�����������ڻ�������б��ֲ���
�𰸣�
������
������
|
|

��ϰ��ϵ�д�
 Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д� ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
�����Ŀ
 һ���¶��£����ݻ�Ϊ1L���ܱ������У��������¹�ϵ��xH2O��g��?��H2O��x��g������Ӧ�������������ʵ�����ʱ��仯��ϵ��ͼ������˵������ȷ���ǣ�������
һ���¶��£����ݻ�Ϊ1L���ܱ������У��������¹�ϵ��xH2O��g��?��H2O��x��g������Ӧ�������������ʵ�����ʱ��仯��ϵ��ͼ������˵������ȷ���ǣ�������| A��x=3 | ||
| B�����¶��£���Ӧ��ƽ�ⳣ��Ϊ0.125L3/mol3 | ||
| C��ƽ��ʱ��������ƽ��Ħ��������33.3g/mol | ||
D��t1ʱ�̣������¶Ȳ��䣬�ٳ���1mol H2O��g�������´ﵽƽ��ʱ��
|
 һ���¶��£����ݻ�Ϊ1L���ܱ������ڷ���2mol N2O4��8mol NO2���������·�Ӧ��2NO2������ɫ��?N2O4����ɫ������H��0������Ӧ��NO2��N2O4�����ʵ����淴Ӧʱ��仯��������ͼ��������Ҫ������
һ���¶��£����ݻ�Ϊ1L���ܱ������ڷ���2mol N2O4��8mol NO2���������·�Ӧ��2NO2������ɫ��?N2O4����ɫ������H��0������Ӧ��NO2��N2O4�����ʵ����淴Ӧʱ��仯��������ͼ��������Ҫ������ һ���¶��£����ݻ�ΪV L���ܱ������н��з�Ӧ��M��N������������ʵ�����ʱ��ı仯������ͼ��ʾ��
һ���¶��£����ݻ�ΪV L���ܱ������н��з�Ӧ��M��N������������ʵ�����ʱ��ı仯������ͼ��ʾ�� M
M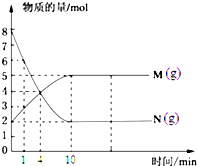 һ���¶��£����ݻ�Ϊ100L�Ķ����ܱ������н���ij��ѧ��Ӧ����Ӧ��ϵ�и���ֵ����ʵ�����ʱ��ı仯������ͼ��ʾ��
һ���¶��£����ݻ�Ϊ100L�Ķ����ܱ������н���ij��ѧ��Ӧ����Ӧ��ϵ�и���ֵ����ʵ�����ʱ��ı仯������ͼ��ʾ��