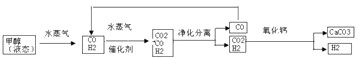
��Ŀ����
��13�֣� ��ҵԭ�ϼ״�������ˮ������Ӧ������������Ӧ����ʽ���£�
CH3OH(g)��H2O(g) ![]() CO2(g)��3H2(g) ��H > 0
CO2(g)��3H2(g) ��H > 0
��1��һ�������£������Ϊ2 L�ĺ����ܱ������г���1 mol CH3OH(g)��3 mol H2O(g)��20 s��û�������ѹǿ�Ƿ�Ӧǰ��1.2�������ü״���ʾ�ķ�Ӧ���� ��
��2����ͼ��P�ǿ�����ƽ�л����Ļ������ر�K������ͬ�¶��£���A�����г���1 mol CH3OH��2 mol H2O(g)����B�����г���1.2 mol CH3OH(g)��2.4 mol H2O(g)���������ֱ���������Ӧ����֪����ʼʱ����A��B�������Ϊa L���Իش�

�� ��Ӧ�ﵽƽ��ʱ������B�����Ϊ1.5 a L��B������CH3OHת����Ϊ ��A��B��������H2O(g)������ٷֺ����Ĵ�С��ϵΪB A����������������������� ��
�� ����K��һ��ʱ������´ﵽƽ�⣬����B�����Ϊ L����ͨ��������������Բ��ƣ��Ҳ������¶ȵ�Ӱ�죩��
��3�������ĸ�ѡ������λѧ����ѧϰ��ѧ��Ӧ�����뻯ѧ��Ӧ���Ժ���ϵ��������ʵ���������Ŀ���������Ϊ����ȷ����_______
A.��ѧ��Ӧ�������ۿ�ָ��������һ��ʱ���ڿ����Ʒ
B.��Ч��ײ���ۿ�ָ���������ԭ�ϵ�ת����
C.��������ԭ����ָ������ʹ������ԭ�϶����Ʒ
D.��ȷ���û�ѧ��Ӧ���ʺͻ�ѧ��Ӧ�ȶ�����������������ۺϾ���Ч��
��4)�״����л����ڿ�����ȼ��ʱ����ȼ�ղ���ֿ��ܻ����CO����Ⱦ����������������Ʒ�Ӧ2CO��2C��O2����H��0����S��0��������CO����Ⱦ�������ж��Ƿ���в�˵�����ɣ�________________��
��1�� v(CH3OH)=0.01mol(L��1•s��1) ��2�֣�
��2�� �� CH3OHת����Ϊ75%����2�֣� �� �� ��2�֣�
�� 1.75a L ��2�֣�
��3��B ��2�֣�
��4�������У�1�֣���
�÷�Ӧ��һ���������ؼ��ķ�Ӧ���κ�����²����Է����� ����2�֣�
 ��������������������ϵ�д�
��������������������ϵ�д�