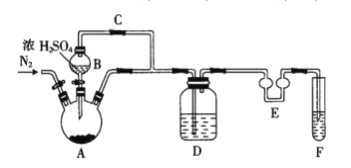
��Ŀ����
����Ŀ���⡢̼��������������ͭ��Ԫ�ؼ��仯���������ǵ��ճ����������Ź㷺����;���ش��������⣺
��1��д����̬ͭԭ�ӵļ۵����Ų�ʽ___��
��2��ʵ������KSCN��Һ������Fe3+��C��N��O�ĵ縺���ɴ�С��˳��Ϊ___(��Ԫ�ط��ű�ʾ)��һ�������£�SCN-��MnO2��Ӧ�ɵõ�(SCN)2����д��(SCN)2�Ľṹʽ___��
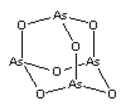
��3��FeCl3���۵�Ϊ306�棬�е�Ϊ315��FeCl3�ľ���������__��FeSO4������������SO42-�����幹����__��
��4��CH3OH������Oԭ�ӵ��ӻ���ʽΪ___�����ǣ�H-C-H___H-O-C��������<������>������=����CH3OH����H2O������Ȼ��ܵ�ԭ����___��
��5����֪C60���ӽṹ��C60����ʾ��ͼ(��ͼI��ͼ����ʾ)��
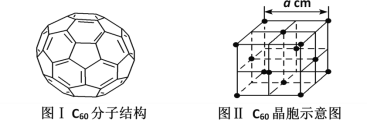
��һ��C60�����к��������ĸ���Ϊ__����ÿ��C60���Ӿ����������ȵ�C60������__����C60������ܶȵı���ʽΪ__g��cm-3��
���𰸡�3d104s1 O��N��C N��C��S��S��C��N ���Ӿ��� ���������� sp3 > ���γɷ��Ӽ���� 90 12 ![]() ��
��![]()
��������
(1)ͭΪ29��Ԫ�أ���̬ͭԭ�Ӻ�����29�����ӣ���۵����Ų�ʽΪ3d104s1���ʴ�Ϊ��3d104s1��
(2) ͬһ����Ԫ�أ��������ҵ縺��������ǿ����C��N��O�ĵ縺���ɴ�С��˳��O��N��C��(SCN)2��S��C��S��S�ֱ��γ�һ�Թ��õ��Ӷԣ�C��N�γ�3�Թ��õ��Ӷԣ���(SCN)2�ĽṹʽΪN��C��S��S��C��N���ʴ�Ϊ��O��N��C��N��C��S��S��C��N��
(3)��ΪFeCl3���۵�Ϊ306�����е�Ϊ315��������FeCl3�Ƿ��Ӿ��壬SO42-��Sԭ�ӳɼ����Ӷ���![]() ���µ��Ӷԣ���SO42-�����幹�������������Σ��ʴ�Ϊ�����Ӿ��壻���������Σ�
���µ��Ӷԣ���SO42-�����幹�������������Σ��ʴ�Ϊ�����Ӿ��壻���������Σ�
(4) CH3OH������Oԭ����C��Hԭ���γ�2���Ҽ�����2���µ��Ӷԣ����ӻ���ʽΪsp3�ӻ����йµ��ӶԵĶԳɼ����ӵ��ų����ô���С�����Լ���H-C-H>H-O-C����Ϊ�״���ˮ�����γɷ��Ӽ����������CH3OH����H2O������Ȼ��ܣ��ʴ�Ϊ��sp3��>�����γɷ��Ӽ������
(5)����C60���ӽṹ��C60������1��̼ԭ����2��C��C����1��C=C�������ݾ�̯����һ��̼ԭ���������еĦҼ��ĸ���Ϊ![]() ����һ��C60�����к��������ĸ���Ϊ
����һ��C60�����к��������ĸ���Ϊ![]() ��������C60�ľ����ṹ����C60�����C60������4�����м���4����������4��������ÿ��C60���Ӿ����������ȵ�C60������12����һ��C60�ľ����У�C60�ĸ���Ϊ
��������C60�ľ����ṹ����C60�����C60������4�����м���4����������4��������ÿ��C60���Ӿ����������ȵ�C60������12����һ��C60�ľ����У�C60�ĸ���Ϊ![]() �����ݹ�ʽ
�����ݹ�ʽ![]() ���ɵþ���������Ϊ
���ɵþ���������Ϊ![]() g���־��������Ϊa3cm3����
g���־��������Ϊa3cm3����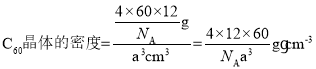 ���ʴ�Ϊ��90��12��
���ʴ�Ϊ��90��12��![]() ��
��![]() ��
��