��Ŀ����
2���ɶ�����Ԫ����ɵĻ�����X��ij����ҩ����Ч�ɷ֣���ͬѧ��̽��X����ɣ��������ϣ����ɶ�����Ԫ����ɵĿ���ҩ����Ч�ɷ���̼�����ơ�̼��þ����������������þ��������������ʽ̼��þ������Al3+��pH=5.0ʱ������ȫ��Mg2+��pH=8.8ʱ��ʼ��������pH=11.4ʱ������ȫ��ʵ����̣�
������X��ĩ�м���������ᣬ������ɫ��ζ����A���õ���ɫ��Һ��
���ò�˿պȡ����I�����õ���Һ���ڻ��������գ���ɫ���森
����I�����õ���Һ�еμӰ�ˮ������pH��5��6��������ɫ����B�����ˣ�
���������B�мӹ���NaOH��Һ������ȫ���ܽ⣮
��������еõ�����Һ�еμ�NaOH��Һ������pH��12���õ���ɫ����C��
��1��A���ӵĵ���ʽΪ��
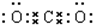 ��C�Ļ�ѧʽΪMg��OH��2
��C�Ļ�ѧʽΪMg��OH��2��2��ʵ���֮ǰҪ�Բ�˿���д��������������Dz�˿��ϴϡ���Ტ�ھƾ��ƻ����������ظ�2-3��
��3���ڢ�ʵ������������Ӧ�����ӷ���ʽ��Al3++3NH3•H20�TAl��OH��3��+3NH4+
��4������B�ܽ�����ӷ���ʽ��Al��OH��3+OH-�TAlO2-+2H2O
��5��������n��A����n��B����n��C��=1��2��3����X�Ļ�ѧʽ��Mg3Al2��OH��10CO3��
���� ������X��ĩ�м���������ᣬ������ɫ��ζ����A����Ͽ���ҩ����Ч�ɷ֣�֪������ΪCO2��
��X��һ������Na����ΪNa����ɫΪ��ɫ��
���������Ϣ֪����pH��5��6ʱ���ɵİ�ɫ����ΪAl��OH��3��
�����������NaOH��Һ������B��ȫ�ܽ⣬���ӷ���ʽΪ��Al��OH��3+OH-�TAlO2-+2H2O��
��������NaOH��Һ����pH��12���а�ɫ���������������CΪMg��OH��2��
��1��A����ΪCO2��������̼�д�������̼��˫��������CΪMg��OH��2��
��2����ɫ��Ӧ��ϡ����ϴ����˿�����ղ�˿���ٽ�����ɫ��Ӧ��
��3�������ӺͰ�ˮ��Ӧ��������������
��4�����������������ԣ����ڹ���NaOH��Һ��
��5������ԭ���غ��Լ�����غ��ƶ�X�Ļ�ѧʽ��
��� �⣺��1���������м���������ᣬ��������������̼���Ρ�̼�����κ����ᷴӦ���ɶ�����̼������X��ĩ�м���������ᣬ������ɫ��ζ����A��������ΪCO2��������̼Ϊֱ���ͽṹ�������д�������̼��˫����������̼�ĵ���ʽΪ��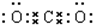 ������NaOH��Һ����pH��12���а�ɫ���������������CΪMg��OH��2��
������NaOH��Һ����pH��12���а�ɫ���������������CΪMg��OH��2��
�ʴ�Ϊ��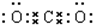 ��Mg��OH��2��
��Mg��OH��2��
��2����ɫ��Ӧ��ϡ����ϴ����˿��ȥ���ʣ���ϴ��Ϊ��ϴȥ��������Ĥ��������ϴ��֮��ͨ�����ھƾ��ƻ��������գ���Ϊ�����Ȼ����ӷ��������������Ӿ�һ���ӷ������������ǣ���˿��ϴϡ���Ტ�ھƾ��ƻ����������ظ�2-3�Σ�
�ʴ�Ϊ����˿��ϴϡ���Ტ�ھƾ��ƻ����������ظ�2-3�Σ�
��3������pH��5��6ʱ���ɵİ�ɫ����ΪAl��OH��3��NH3•H20Ϊ������ʣ����ӷ���ʽ��ӦдΪ��ѧʽ��
�ʴ�Ϊ��Al3++3NH3•H20�TAl��OH��3��+3NH4+��
��4��Al��OH��3Ϊ�����������������ǿ��������NaOH��Һ��Al��OH��3������ȫ�ܽ⣬���ӷ���ʽΪ��Al��OH��3+OH-�TAlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-�TAlO2-+2H2O��
��5������n��CO2����n[Al��OH��3]��n[Mg��OH��2]=1��2��3����CO32-��Al3+��Mg2+�����ʵ���֮��Ϊ1��2��3����ϵ���غ㣬��CO32-��Al3+��Mg2+��OH-�����ʵ���֮��Ϊ1��2��3��10����XΪMg3Al2��OH��10CO3��
�ʴ�Ϊ��Mg3Al2��OH��10CO3��
���� ���⿼�鿹��ҩ�ɷֵ�̽��ʵ�飬��Ŀ��Ϊ�ۺϣ��Ѷ��еȣ������״���Ϊ�ڣ�5���⣬���������غ㶨�ɽ��

 �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�| A�� | ��ȩ�Ľṹʽ��HCHO | B�� | ������ӵı���ģ�ͣ� | ||
| C�� | ���Ȼ�̼���ӵĵ���ʽ��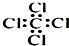 | D�� | 2-�һ�-1��3-����ϩ���ӵļ���ʽ��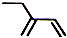 |
| A�� | Ԫ�����ڱ����߸�������Զ����ı� | |
| B�� | Ԫ�����ڱ��оŸ����У���Ϊ�߸����� | |
| C�� | Ԫ�����ڱ���ʮ�˸����У���Ϊʮ������ | |
| D�� | Ԫ�����ڱ���ʮ�˸����У���Ϊʮ�˸��� |
| A�� | �ð�ˮ����Al3+��Mg2+��Ag+ | |
| B�� | ��Ba�� NO3��2��Һ����Cl-��$SO_4^{2-}$��$CO_3^{2-}$ | |
| C�� | �ú˴Ź���������l-������2-����� | |
| D�� | ��KMnO4������Һ����CH3CH�TCHCH2OH��CH3CH2CH2CHO |
| A�� | ����ɫ��NO2��ѹ����ɫ�ȱ����ٱ�dz | |
| B�� | H2��I2��HI��������ѹ����ɫ���� | |
| C�� | �ϳɰ�ʱ���¡���ѹ�Ժϳɰ����� | |
| D�� | ������Һϡ��ʱ����ҺpH���� |
| A�� | ���Ӽ�����ʹ���������ӽ�ϳɻ�����ľ������� | |
| B�� | �ɷǽ���Ԫ����ɵĻ����ﲻһ���ǹ��ۻ����� | |
| C�� | ���ӻ�������ܺ����ۼ������ۻ������в������Ӽ� | |
| D�� | ͬ���ڷǽ���Ԫ���������Ӧˮ��������Դ�����������ǿ |
| A�� | �¶���ͬ�������ͬ��O2��N2 | B�� | ������ȡ��ܶȲ��ȵ�N2��C2H4 | ||
| C�� | �����ͬ���ܶȲ��ȵ�CO��C2H4 | D�� | ѹǿ��ͬ�������ͬ��O2��H2 |
��֪���ٹ��������ɵ��м����ʸ����Լ�����ˮ�ⷴӦ��
�ڲ���������ʵķе����£�
| ���� | �е�/�� |
| �����״� | 380 |
| ���� | 34.6 |
| �屽 | 156.2 |
��ش��������⣺
��1��װ���в�������B������Ϊ�����ܣ�װ����ˮCaCl2������A�������Ƿ�ֹ�����е�ˮ��������װ�ã���������Լ�ˮ�⣮
��2��װ���еμ�Һ��δ����ͨ��Һ©�����õ�Һ©����������ƽ��ѹǿ��ʹ©����Һ��˳�����£�
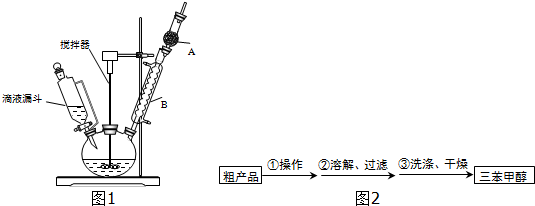
��3���Ƶõ������״��ֲ�Ʒ�к������ѡ��屽���Ȼ�淋����ʣ����������ͼ2�ᴿ���������У������ٵ���������������ϴ��Һ���ѡ��a������ĸ��ţ���
a��ˮ b������ c���Ҵ� d����
�����Ʒ�Ѿ�ϴ�Ӹɾ��IJ���Ϊȡ�������һ��ϴ��Һ���Թ��У��μ���������Һ�����������ɣ����Ѿ�ϴ�Ӹɾ�����֮��δϴ�Ӹɾ���
��4�����Ȳⶨ����ȡ2.60g��Ʒ�����������Һ���������������ƣ��������Ʋ���Ӧ������ַ�Ӧ������ɵ������ڱ�״���µ����Ϊ100.80mL�����Ʒ�������״�����������Ϊ90%��
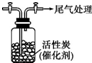 ijѧϰС������SO2���л�ԭ�ԣ��Ʋ�SO2�ܱ�Cl2��������SO2Cl2���������ϣ�SO2Cl2�ڳ�����Ϊ��ɫҺ�壬����ˮ�⣬����ʪ���������������
ijѧϰС������SO2���л�ԭ�ԣ��Ʋ�SO2�ܱ�Cl2��������SO2Cl2���������ϣ�SO2Cl2�ڳ�����Ϊ��ɫҺ�壬����ˮ�⣬����ʪ���������������