��Ŀ����
7��ijѧϰС��Ӻ�������ȡ���ʵ⣬ʵ�鲽�����£����ʴ��й����⣮��1��ʵ���ұ��պ�������Ҫ���������е�cdef������ţ���
a���Թ� b���ձ� c������ d�������� e�������ż� f���ƾ���
��2�������ú����Ҽ�ˮ�ܽ⡢���ˣ�����IJ���������©�����ձ��Ͳ�������
��3������Һ�м���H202��ʹ��Ԫ��ת��Ϊ�ⵥ�ʣ�
��4��С���Ա��CCl4��ȡ��ˮ�еĵ⣬�ڷ�Һ©���У��²�Һ�����ɫ�����Ǵ�Һ©��������ȴδ��Һ�����£�ԭ������Ƿ�Һ©���Ͽڻ���С��δ�������ͨ��
���� ��1�����պ�����Ҫ�������У��ƾ��ơ����żܡ������Ǽ�������
��2�������ú����Ҽ�ˮ�ܽ⡢���˵IJ������ܽ⡢����װ�ã���Ҫ©�������������ձ���
��4�����Ȼ�̼���ܶȴ���ˮ�Һ�ˮ�����ܣ����Ȼ�̼����ȡ�⣬�����л������·���ˮ���Ϸ��������Һ©���Ͽڻ���С��δ�������ͨ����Һ�岻��������
��� �⣺��1�����պ�����Ҫ�������У��ƾ��ơ����żܡ������Ǽ���������ѡcdef��
�ʴ�Ϊ��cdef��
��2�������ú����Ҽ�ˮ�ܽ⡢���˵IJ������ܽ⡢����װ�ã���Ҫ©�������������ձ���
�ʴ�Ϊ��©�����ձ��Ͳ�������
��4�����Ȼ�̼���ܶȴ���ˮ�Һ�ˮ�����ܣ����Ȼ�̼����ȡ�⣬�����л������·���ˮ���Ϸ���������Ȼ�̼��Һ����ɫ�������Һ©���Ͽڻ���С��δ�������ͨ����Һ�岻��������
�ʴ�Ϊ���ϣ���Һ©���Ͽڻ���С��δ�������ͨ��
���� ���⿼�麣ˮ��Դ�ۺ����ã����ؿ���ѧ���Ի�ѧʵ������������������գ���ȷʵ��ԭ���ǽⱾ��ؼ���֪����ȡ����ѡȡ����֪�������������ã���Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
17������˵����ȷ���ǣ�������
| A�� | �������DZ����� | |
| B�� | ��������е�����Cԭ�ӹ��ߣ���C��H�˸�ԭ�ӹ��� | |
| C�� | C3H8�����е�����̼ԭ�ӿ��ܹ��ߣ������е�ԭ�Ӳ����ܹ��� | |
| D�� | C20H42һ���������� |
18��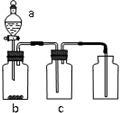 ����ͼװ����ȡ���ᴿ���ռ����е��������壨a��b��c��ʾ��Ӧ�����м�����Լ��������п��е��ǣ�������
����ͼװ����ȡ���ᴿ���ռ����е��������壨a��b��c��ʾ��Ӧ�����м�����Լ��������п��е��ǣ�������
 ����ͼװ����ȡ���ᴿ���ռ����е��������壨a��b��c��ʾ��Ӧ�����м�����Լ��������п��е��ǣ�������
����ͼװ����ȡ���ᴿ���ռ����е��������壨a��b��c��ʾ��Ӧ�����м�����Լ��������п��е��ǣ�������| ���� | a | b | c | |
| A | NO2 | Ũ���� | ͭƬ | NaOH��Һ |
| B | SO2 | Ũ���� | Cu | Ũ���� |
| C | O2 | ˫��ˮ | �������� | ��ʯ�� |
| D | CO2 | ϡ���� | CaCO3 | ����NaHCO3��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
15���ֱ���ȫ���������ʵ���Ũ�ȵ�KCl��CaCl2��AlCl3��Һ�е�Cl-��������ͬ���ʵ���Ũ��ΪAgNO3��Һ�����֮��Ϊ3��2��1����KCl��CaCl2��AlCl3��Һ�����֮��Ϊ��������
| A�� | 6��3��2 | B�� | 1��1��1 | C�� | 9��3��1 | D�� | 3��2��1 |
12����106g̼���ƺ�����ͬ��ԭ���������������Ϊ��������
| A�� | 49.0g | B�� | 73.5g | C�� | 98.0g | D�� | 147g |
19��������˵������ʽΪC4H6��ij����1-��Ȳ������1��3-����ϩ����ʵ���ǣ�������
| A�� | ����ԭ�Ӳ���ͬһƽ�� | |
| B�� | ��ʹ���Ը��������Һ��ɫ | |
| C�� | ȼ��ʱ��Ũ�� | |
| D�� | ��������ˮ��Ӧʱ����������ֻ��2��̼ԭ��������ԭ�� |
16����������ʹ�úͲ���������˵����ȷ���ǣ�������
| A�� | ����ƿ��ʹ��ǰһ��Ҫ��©��ϴ�Ӳ���� | |
| B�� | ʹ�ý�ͷ�ι�ʱ�����������Ҳ�ɰѵιܲ��뵽��Һ�� | |
| C�� | ȡ�����У�����Ҫ��Һ©���IJ��������Է�ֹ��©�� | |
| D�� | ����ʱ����ȴˮ������������һ�˽��룬��������Ч���� |
3��K2Cr2O7��Ƥ�﹤ҵ������Ҫ����;��ͬʱҲ�Dz�����Ⱦ����Ҫԭ���������Ҫ�ɷֿɱ�ʾΪFeO•Cr2O3��������MgO��Al2O3��Fe2O3�����ʣ�ͼ1���Ը�����Ϊԭ���Ʊ��ظ���أ�K2Cr2O7��������ͼ��

��֪����4FeO•Cr2O3+8Na2CO3+7O2$\stackrel{750��}{��}$8Na2CrO4+2Fe2O3+8CO2����
��Na2CO3+Al2O3$\stackrel{750��}{��}$2NaAlO2+CO2���� ��Cr2O72-+H2O?2CrO42-+2H+
��������ش��������⣺
��1������X����Ҫ����Fe2O3��MgO����д��ѧʽ����Ҫ����ữ��������Һ��pH�Ƿ����4.5��Ӧ��ʹ��pH�ƻ���pH��ֽ����д�������Լ����ƣ���
��2���ữ�������ô��������ҺpH��5����Ŀ����ʹCrO42-ת��ΪCr2O72-��
��3�������������ʵ��ܽ�����ݣ�����������Ӧ�Ļ�ѧ����ʽ�ǣ�Na2Cr2O7+2KCl��K2Cr2O7��+2NaCl��
�÷�Ӧ����Һ���ܷ�����������K2Cr2O7���ܽ�ȱ�Na2Cr2O7С��������������K2Cr2O7���ܽ����С����
��4������ƷY��Ҫ��������������������þ���������ܻ����P���������ʣ���ȷ����Y���������������ķ����dz�ȡng��Ʒ���������NaOH��Һ����д�Լ������ܽ⡢���ˡ���ͨ�����������̼����д�Լ����������ա���ȴ���������ø������m g��������Ʒ��������������������Ϊ$\frac{m}{n}$���ú�m��n�Ĵ���ʽ��ʾ����
��5���õ�ⷨ��ȥ��Cr2O72-�����Է�ˮ�����ǣ���Fe���缫��⺬Cr2O72-�����Է�ˮ�����Ž���У�������������ҺpH���ߣ�����Cr��OH��3��������Fe���缫��ԭ��Ϊ����ϵ缫��Ӧ���ͣ���ͬ��������ӦΪFe-2e-=Fe2+���ṩ��ԭ��Fe2+��������������ҺpH���ߵ�ԭ����2H++2e-=H2���������ӷŵ�ʹOH-Ũ������

��֪����4FeO•Cr2O3+8Na2CO3+7O2$\stackrel{750��}{��}$8Na2CrO4+2Fe2O3+8CO2����
��Na2CO3+Al2O3$\stackrel{750��}{��}$2NaAlO2+CO2���� ��Cr2O72-+H2O?2CrO42-+2H+
��������ش��������⣺
��1������X����Ҫ����Fe2O3��MgO����д��ѧʽ����Ҫ����ữ��������Һ��pH�Ƿ����4.5��Ӧ��ʹ��pH�ƻ���pH��ֽ����д�������Լ����ƣ���
��2���ữ�������ô��������ҺpH��5����Ŀ����ʹCrO42-ת��ΪCr2O72-��
��3�������������ʵ��ܽ�����ݣ�����������Ӧ�Ļ�ѧ����ʽ�ǣ�Na2Cr2O7+2KCl��K2Cr2O7��+2NaCl��
| ���� | �ܽ��/��g/100gˮ�� | ||
| 0��C | 40��C | 80��C | |
| KCl | 28 | 40.1 | 51.3 |
| NaCl | 35.7 | 36.4 | 38 |
| K2Cr2O7 | 4.7 | 26.3 | 73 |
| Na2Cr2O7 | 163 | 215 | 376 |
��4������ƷY��Ҫ��������������������þ���������ܻ����P���������ʣ���ȷ����Y���������������ķ����dz�ȡng��Ʒ���������NaOH��Һ����д�Լ������ܽ⡢���ˡ���ͨ�����������̼����д�Լ����������ա���ȴ���������ø������m g��������Ʒ��������������������Ϊ$\frac{m}{n}$���ú�m��n�Ĵ���ʽ��ʾ����
��5���õ�ⷨ��ȥ��Cr2O72-�����Է�ˮ�����ǣ���Fe���缫��⺬Cr2O72-�����Է�ˮ�����Ž���У�������������ҺpH���ߣ�����Cr��OH��3��������Fe���缫��ԭ��Ϊ����ϵ缫��Ӧ���ͣ���ͬ��������ӦΪFe-2e-=Fe2+���ṩ��ԭ��Fe2+��������������ҺpH���ߵ�ԭ����2H++2e-=H2���������ӷŵ�ʹOH-Ũ������
