��Ŀ����
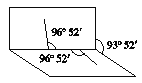
����Ŀ��ij������������Al��(NH4)2SO4��MgCl2��FeCl2��AlCl3�е����ֻ�����ɣ��ֶԸû����������ʵ�飬����������й�������ͼ��ʾ(������������ѻ���ɱ�״���µ����)������˵���в���ȷ����( )

A���ù�����һ��û��FeCl2�����ܺ���AlCl3
B���ù����к���2��70 g Al
C���ù����к���6��60 g (NH4)2SO4
D���ù����к���4��75 g MgCl2
���𰸡�A
��������
�������������Ϲ����м������Ũ��NaOH��Һ���ܹ������������Al��(NH4)2SO4 ������ֱ���H2��NH3��������ͨ����ʯ�Ҹ������������䣬��ͨ��Ũ���ᣬ���������С�Ҳ�Ϊ�㣬���Կ�֪���ɵ�����ΪH2��NH3 ������ֱ�Ϊ3��36L��2��3L��ԭ����������һ����Al��(NH4)2SO4 ����������Էֱ�����m(Al)=2��70g��m[(NH4)2SO4]=6��60g����������а�ɫ�������ɣ��Ұ�ɫ�������ò���ɫ����һ��û��FeCl2������ΪMg(OH)2�� 2��9g���ɴ˿ɼ����m(MgCl2)=4��75g���ɴ�m(Al)+m[(NH4)2SO4] +m(MgCl2)= 2��70g +6��60g +4��75g=14��05g�����Թ���������ֻ�����������ʣ�һ��û��AlCl3 ������A����ѡA��

 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�