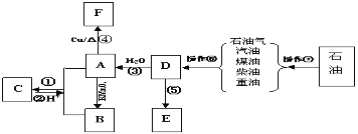
��Ŀ����
����Ŀ��A��B��C��D���Ƕ�����Ԫ�أ�ԭ�Ӱ뾶D>C>A>B������A��B����ͬһ���ڣ�A��C����ͬһ���塣Cԭ�Ӻ�������������A��Bԭ�Ӻ���������֮�ͣ�Cԭ��������ϵĵ�������Dԭ��������������4�����Իش�
��1��������Ԫ�طֱ��ǣ�A____��B___��C___��D____��
��2��������Ԫ�����ڳ��³�ѹ�µ�Һ̬����̬�⻯����ȶ����ɴ��С��˳����_____��
��3��A��B�γɵ���ԭ�ӷ��ӵĵ���ʽ��___��B��D�γ�ԭ�Ӹ�����Ϊ1��1�Ļ�����ĵ���ʽ��_____��
��4��AԪ��ij��������DԪ��ij�����ﷴӦ���ɵ��ʵĻ�ѧ����ʽ��_______��
���𰸡�(1)̼ �� �� �� ����1�֣� (2)H2O>CH4>SiH4 (2��)
(3)![]() ����1�֣�
����1�֣�
(4)2CO2��2Na2O2��2Na2CO3��O2 (2��)
��������
����A��B��C��D���Ƕ�����Ԫ�أ�ԭ�Ӱ뾶D��C��A��B������A��B����ͬһ���ڣ�A��C����ͬһ���壬����Ԫ�������ڱ��еĴ������λ��Ϊ��![]() ��A��C����ͬһ���壬�������������8��Cԭ�Ӻ�������������A��Bԭ�Ӻ���������֮�ͣ���B��������Ϊ8��BΪOԪ�أ�Cԭ��������ϵĵ�������Dԭ��������������4������C����������Ϊ4��D������������Ϊ1����AΪCԪ�أ�CΪSiԪ�أ�DΪNaԪ�أ���
��A��C����ͬһ���壬�������������8��Cԭ�Ӻ�������������A��Bԭ�Ӻ���������֮�ͣ���B��������Ϊ8��BΪOԪ�أ�Cԭ��������ϵĵ�������Dԭ��������������4������C����������Ϊ4��D������������Ϊ1����AΪCԪ�أ�CΪSiԪ�أ�DΪNaԪ�أ���
��1���������Ϸ�����֪����Ԫ�����Ʒֱ�Ϊ̼�������衢�ơ�
��2���ǽ�����Խǿ���⻯����ȶ���Խǿ���ǽ�������O>C>Si�������⻯����ȶ����ɴ��С��˳����H2O>CH4>SiH4��
��3��̼�����γɵ���ԭ�ӷ����Ƕ�����̼�������ʽ��![]() ���������γɵ�ԭ�Ӹ�����Ϊ1��1�Ļ������ǹ������ƣ������ʽ��
���������γɵ�ԭ�Ӹ�����Ϊ1��1�Ļ������ǹ������ƣ������ʽ��![]() ��
��
��4��������̼����������Ʒ�Ӧ����������̼���ƣ���Ӧ�ķ���ʽΪ2CO2��2Na2O2��2Na2CO3��O2��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����ӦFe��s��+CO2��g��![]() FeO��s��+CO��g����ƽ�ⳣ��ΪK1����ӦFe��s��+H2O��g��
FeO��s��+CO��g����ƽ�ⳣ��ΪK1����ӦFe��s��+H2O��g��![]() FeO��s��+H2��g����ƽ�ⳣ��ΪK2���ڲ�ͬ�¶�ʱK1��K2��ֵ���±���
FeO��s��+H2��g����ƽ�ⳣ��ΪK2���ڲ�ͬ�¶�ʱK1��K2��ֵ���±���
�¶ȣ������¶ȣ� | K1 | K2 |
973 | 1.47 | 2.38 |
1173 | 2.15 | 1.67 |
��1�������¶�Ϊ973Kʱ����ӦCO2��g��+H2��g��![]() CO��g��+H2O��g�� K=__________��
CO��g��+H2O��g�� K=__________��
��2��Ŀǰ��ҵ����һ�ַ�������CO2�������״���CO2(g)��3H2(g) ![]() CH3OH(g)��H2O(g)�������Ϊ1 L�ĺ����ܱ������У�����1 mol CO2��3 mol H2���з�Ӧ��
CH3OH(g)��H2O(g)�������Ϊ1 L�ĺ����ܱ������У�����1 mol CO2��3 mol H2���з�Ӧ��
�ٸ÷�Ӧ�ܹ��Է����е�ԭ����________��
�����д�ʩ����ʹc(CH3OH)�������________��
A�������¶�
B������He(g)��ʹ��ϵѹǿ����
C����H2O(g)����ϵ�з������
D���ٳ���1 mol CO2��3 mol H2
�����¶�T1ʱ������Ӧ�ﵽƽ��ʱ�����n(H2)��2.4 mol�������������䣬���¶�T2ʱ������Ӧ�ﵽƽ��ʱ�����n(CO2)��0.82 mol����T2________T1(������������������������)��
��3��ijʵ�齫һ������CO2��H2����һ��������ܱ������У������ֲ�ͬ�����·�����Ӧ��CO2(g) ��3H2(g) ![]() CH3OH(g)��H2O(g) ��H����49.0 kJ��mol��1�����CH3OH�����ʵ�����ʱ��仯����ͼ��ʾ���ش����⣺
CH3OH(g)��H2O(g) ��H����49.0 kJ��mol��1�����CH3OH�����ʵ�����ʱ��仯����ͼ��ʾ���ش����⣺

������I������Ӧ��ƽ�ⳣ����С��ϵΪK��________K��(������������������������)��
��һ���¶��£����ݻ���ͬ�ҹ̶��������ܱ������У������·�ʽͶ�뷴Ӧ�һ��ʱ���ﵽƽ�⡣
���� | �� | �� |
��Ӧ�� Ͷ���� | 1 mol CO2��3 mol H2 | a mol CO2��b mol H2�� c mol CH3OH(g)��c mol H2O(g) |
������ƽ��������ѹǿΪ��ʼʱ��0.8����Ҫʹƽ������������ͬ��ֵ�Ũ����ȣ�����ʼʱά�ַ�Ӧ������У���c��ȡֵ��ΧΪ________��