��Ŀ����
C��CuO��һ���¶��·�Ӧ��������Cu��Cu2O��CO��CO2����1.20 g C��16.0 g CuO��ϣ������������ȣ������ɵ�����ȫ��ͨ�������ij���ʯ��ˮ����Ӧһ��ʱ����ռ���1.12 L���壨��״���������ɳ���������Ϊ5.00 g������˵����ȷ����
| A����Ӧ��Ĺ��������л�����̼ |
| B����Ӧ��Ĺ�������������Ϊ13.6 g |
| C����Ӧ��Ĺ�������������������ʵ���Ϊ0.05 mol |
| D����Ӧ��Ĺ��������е���Cu������Ϊ12.8 g |
BC
�������������n��C��=0.1mol,n��CuO��=0.2mol.n��CaCO3��=n��CO2��=" 5.00" g��100g/mol= 0.05mol,n��CO��="1.12L��" 22.4L/mol ="0.05mol," n��CO2��+ n��CO��=n��C��=0.1mol���Թ�������̼���ʴ��ڡ������е���Ԫ�ص����ʵ���Ϊn��O��= 2n��CO2��+ n��CO��=0.15mol< n��CuO��=0.2mol,���Թ����л�����0.05mol��Cu2O������Ϊ0.05mol��144g/mol=7.2g,������Cu������Ϊ��0.2mol-0.05mol��2����64g/mol=6.4g.���Թ����������Ϊ7.2g+6.4g="13.6" g.����ȷѡ��ΪB C��
���㣺�������C��CuO��һ���¶��·�Ӧ����ijɷֵ�ȷ����֪ʶ��

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�ȡNa2O��Na2O2��Na2CO3�Ĺ���������������ʵ�飬���¼�¼��������ʵ����
| A������ˮ�У�������ɫ���� |
| B�������̪��Һ�У���Һ�ȱ�����ɫ |
| C���ڸɿ����м��ȵ�400�棬�������� |
| D����SO2��Ӧ����Na2SO4���� |
���й���Na2CO3��NaHCO3�����ʱȽ��У�����ȷ����
| A�����ȶ��ԣ� Na2CO3 > NaHCO3 |
| B������ʱ��ˮ�е��ܽ�ȣ�Na2CO3 > NaHCO3 |
| C����ϡ���ᷴӦ�ľ��ҳ̶ȣ�Na2CO3 > NaHCO3 |
| D���������Ĺ��������������ᷴӦ�ų�CO2��������NaHCO3 > Na2CO3 |
���и����е����������Ӧʱ�����ı䷴Ӧ�������¶ȡ���Ӧ�������ȣ�����ѧ��Ӧ�����ı����
| A��Al������������Һ | B��NaOH��CO2 |
| C��Na��O2 | D��AlCl3��NaOH |
��agþ���Ͻ�Ͷ�뵽x mL 2mol/L�������У�������ȫ�ܽ���ټ���y mL 1mol��L������������Һ���õ��ij������������Ϊ g������˵������ȷ����
g������˵������ȷ����
A��þ���Ͻ������ᷴӦת�Ƶ�������Ϊ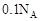 |
B�� |
C��������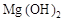 �� ��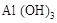 �Ļ���� �Ļ���� |
| D��1.2��a��0.9 |
��һ�����ġ��������Ʒ����5mol/L����200mLǡ����ȫ�ܽ⣬������Һ�������ձ����2��24L������ǡ��ʹ����Fe2��ȫ��ת����Fe3��������Ʒ���ܵĻ�ѧʽ��(����)
| A��Fe2O3 | B��Fe3O4 | C��Fe4O5 | D��Fe5O7 |
ijCuSO4��Fe2��SO4��3��H2SO4�Ļ����Һ100 mL����֪��Һ�������ӵ�Ũ����ͬ��������ˮ�⣩����SO42�� �����ʵ���Ũ��Ϊ6 mol��L��1�������Һ����ܽ����۵�����Ϊ�� ��
| A��5��6 g | B��11��2 g | C��22��4 g | D��33��6 g |

