��Ŀ����
ʵ�����п���������һЩװ�����������ƴ�����CO2���壬���ⶨ�������(�ú�����FeS�Ĵ���ʯ�������Ʊ�CO2����)��
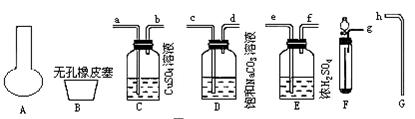
ʵ�鲽�����£�
(1)����Ƥ��B����A��ƿ�ϣ�����Ƥ��������ƿ��ƿ�����Ա����Ƥ����ƿ������ȣ�ȷ����������Ϊw1��
(2)������ȡ�;��������װ�ã�������˳���ǣ�_____��______��______��_____��_____��_____��______��______��
(3)������G������ƿA������_____(��/��)�������ռ�CO2������CO2�Ƿ��Ѿ�����������CO2�ѳ����ķ�����______������Ƥ��������ƿƿ�ڳ�������Ϊw2��
(4)����ƿ��ƿ��ȡ������ƿעˮ������ߣ��ٽ�ˮ������Ͳ����ˮ�����ΪVmL��
(5)��¼��ʱʵ��ʱ����Ϊt���ѹǿΪp����(Rȡ8.314Pa��L��K-1��mol-1)
�ش��������⣺
�ټ���CO2����������ʽ��________��
�����������徻�������ⶨ��CO2����������ֵ��_____(ƫ��/ƫ��)����ԭ����_____��

ʵ�鲽�����£�
(1)����Ƥ��B����A��ƿ�ϣ�����Ƥ��������ƿ��ƿ�����Ա����Ƥ����ƿ������ȣ�ȷ����������Ϊw1��
(2)������ȡ�;��������װ�ã�������˳���ǣ�_____��______��______��_____��_____��_____��______��______��
(3)������G������ƿA������_____(��/��)�������ռ�CO2������CO2�Ƿ��Ѿ�����������CO2�ѳ����ķ�����______������Ƥ��������ƿƿ�ڳ�������Ϊw2��
(4)����ƿ��ƿ��ȡ������ƿעˮ������ߣ��ٽ�ˮ������Ͳ����ˮ�����ΪVmL��
(5)��¼��ʱʵ��ʱ����Ϊt���ѹǿΪp����(Rȡ8.314Pa��L��K-1��mol-1)
�ش��������⣺
�ټ���CO2����������ʽ��________��
�����������徻�������ⶨ��CO2����������ֵ��_____(ƫ��/ƫ��)����ԭ����_____��
(2)g��c��d��a��b��e��f��h
(3)�ϣ�ȼ�ŵ�ľ��������ƿ���Ϸ�������Ϩ��˵��CO2�ѳ���
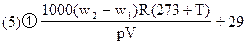
��ƫ�ͣ�CO2��������������H2S��HCl��H2O(g)��CO2������С
(3)�ϣ�ȼ�ŵ�ľ��������ƿ���Ϸ�������Ϩ��˵��CO2�ѳ���
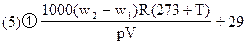
��ƫ�ͣ�CO2��������������H2S��HCl��H2O(g)��CO2������С
(1)w1ӦΪ��ƿ����Ƥ���Լ�ƿ�ڿ�����������
(2)��װ��F��ȡCO2���壬���ֱ�װ��D��C��E�Գ�ȥHCl��H2S�Լ�ˮ���������ʣ��������ƿA���ռ���
(3)CO2�ȿ����أ����������������ռ�������CO2�ķ���ͨ����ʹȼ�ŵ�ľ��Ϩ��ķ�����w2ΪCO2���塢��Ƥ������ƿ����������
(4)VΪ�ռ���CO2�������
(2)��װ��F��ȡCO2���壬���ֱ�װ��D��C��E�Գ�ȥHCl��H2S�Լ�ˮ���������ʣ��������ƿA���ռ���
(3)CO2�ȿ����أ����������������ռ�������CO2�ķ���ͨ����ʹȼ�ŵ�ľ��Ϩ��ķ�����w2ΪCO2���塢��Ƥ������ƿ����������
(4)VΪ�ռ���CO2�������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


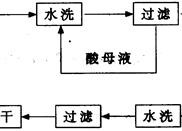
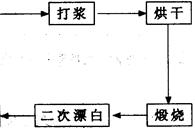

 CaCl2��2NH3����2H2O���ʣ�
CaCl2��2NH3����2H2O���ʣ�