��Ŀ����
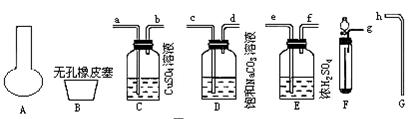
��������ʾ��A��B��C����װ�ö�����ȡ�屽������ϸ��������װ�ã�Ȼ��ش��������⣺
(1)д������װ���ж������ķ�Ӧ�Ļ�ѧ����ʽ____________________��____________________��д��B���Թ��л������ķ�Ӧ�Ļ�ѧ����ʽ____________________��
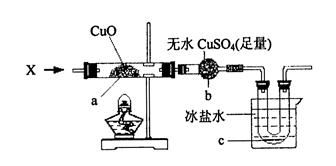
(2)װ��A��C�г����ܵ�������____________________��
(3)B��Cװ�������Ӻã��������������Լ��飬Ҳװ���˺��ʵ�ҩƷ��������Ҫʹ��Ӧ��ʼ����BӦ���еIJ�����__________________����CӦ���еIJ�����____________________��
(4)A�д��ڼ�װҩƷ�ͼ�ʱ�ܷ��ì�ܣ������ʵ��������ɵIJ��������____________________��
(5)B�в�����˫�����չܣ���������________________����Ӧ��˫����п��ܳ��ֵ�������________________��˫�����Һ�岻��̫�࣬ԭ����______________________________________________________��
(6)Bװ��Ҳ�����������Ե�ȱ�㣬ʹʵ���Ч�����û����������У�������ȱ����________________��________________.
(1)д������װ���ж������ķ�Ӧ�Ļ�ѧ����ʽ____________________��____________________��д��B���Թ��л������ķ�Ӧ�Ļ�ѧ����ʽ____________________��
(2)װ��A��C�г����ܵ�������____________________��
(3)B��Cװ�������Ӻã��������������Լ��飬Ҳװ���˺��ʵ�ҩƷ��������Ҫʹ��Ӧ��ʼ����BӦ���еIJ�����__________________����CӦ���еIJ�����____________________��
(4)A�д��ڼ�װҩƷ�ͼ�ʱ�ܷ��ì�ܣ������ʵ��������ɵIJ��������____________________��
(5)B�в�����˫�����չܣ���������________________����Ӧ��˫����п��ܳ��ֵ�������________________��˫�����Һ�岻��̫�࣬ԭ����______________________________________________________��
(6)Bװ��Ҳ�����������Ե�ȱ�㣬ʹʵ���Ч�����û����������У�������ȱ����________________��________________.
��1��2Fe+3Br2 2FeBr3
2FeBr3

HBr+AgNO3 AgBr��+HNO3
AgBr��+HNO3
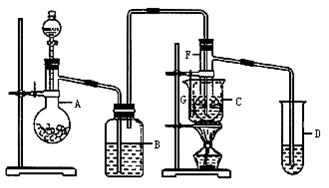
��2������������HBr�������������� ��Br2������
��Br2������
��3����ת��Һ©��������ʹBr2�ͱ��Ļ����ε���������������ʹFe���ص���������ͱ���ɵĻ��Һ��
��4��Br2�ͱ��������ݳ�����Ⱦ����
��5�����շ�Ӧ����HBr�����ݳ���Br2�ͱ�����CCl4����ɫ���ɫ�ױ�����ѹ���Թ���
��6����HBr�ݳ����������ͱ��������ܻ�������Ӧ���У�ԭ�������ʵ� ���ܲ���AgNO3��Һ���ײ�������
 2FeBr3
2FeBr3
HBr+AgNO3
 AgBr��+HNO3
AgBr��+HNO3��2������������HBr��������������
 ��Br2������
��Br2��������3����ת��Һ©��������ʹBr2�ͱ��Ļ����ε���������������ʹFe���ص���������ͱ���ɵĻ��Һ��
��4��Br2�ͱ��������ݳ�����Ⱦ����
��5�����շ�Ӧ����HBr�����ݳ���Br2�ͱ�����CCl4����ɫ���ɫ�ױ�����ѹ���Թ���
��6����HBr�ݳ����������ͱ��������ܻ�������Ӧ���У�ԭ�������ʵ� ���ܲ���AgNO3��Һ���ײ�������
��FeBr3�������£�����Һ�巢��ȡ����Ӧ�������屽��HBr��HBr����AgNO3��HNO3�Ļ����Һ�����������ڷ�Ӧ����,�塢���е�ͣ��ӷ�������Ӧ��������װ�ã�����Br2��g��������g�������������ɻ�����Ⱦ������Ӧ���з�������װ�á�

��ϰ��ϵ�д�
 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д� �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д�
�����Ŀ