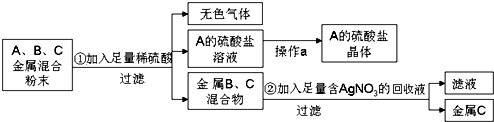
��Ŀ����
7��ij��ѧ��ѧ��ȤС��Ϊ�˲ⶨij��ɽʯ��ʯ��̼��Ƶ�����������ȡʯ��ʯ��Ʒ��������10%��ϡ�������ձ��з�Ӧ������ʯ��ʯ��Ʒ�����ʲ���ϡ���ᷴӦҲ������ˮ�����й�ʵ���������±���| ��Ӧǰ | ��Ӧ�� | ||
| ʵ�� ���� | �ձ���ϡ��������� | ʯ��ʯ��Ʒ������ | �ձ������л��������� |
| 134.4g | 10g | 141.1g | |
��1����ʯ��ʯ��̼��Ƶ�����������
��2���μӷ�Ӧ10%�������������
���� ������̼������=134.4g+10g-141.1g=3g�����ݷ�Ӧ�ķ���ʽCaCO3+2HCl=CaCl2+H2O+CO2���ɼ���̼��Ƶ����������������������������ݷ���ʽ��������HCl����������������������������������Դ˽��
��� �⣺������̼������=134.4g+10g-141.1g=3g
��10gʯ��ʯ��̼��Ƶ�����Ϊx���μӷ�Ӧ��HCl����Ϊy��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 44
x y 3.3 g
100��x=44��3.3g��
x=7..5g��
73��y=44��3.3g��
y=5.475g��
CaCO3%=7.5g��10g��100%=75%��
���������=5.475g��10%=54.75 g��
�𣺣�1����ʯ��ʯ��̼��Ƶ���������Ϊ75%��
��2���μӷ�Ӧ10%�����������Ϊ54.75 g��
���� ������Ҫ����ѧ�����������غ㶨�ɡ���ѧ����ʽ������������ʽ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
18�����и�����̬�⻯���ȶ�����ǿ������˳�����е��ǣ�������
| A�� | SiH4��PH3��H2S��HCl | B�� | HF��HCl��HBr��HI | ||
| C�� | PH3��H2S��HCl��HF | D�� | NH3��PH3��AsH3��HF |
12������ȼ�ϵ����һ������Դ��ͼΪ����ȼ�ϵ��ʾ��ͼ������˵������ȷ���ǣ�������
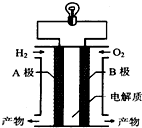

| A�� | ������B��ͨ����������A�� | |
| B�� | A���Ǹ�����B�������� | |
| C�� | ��װ���ܽ���ѧ��ת��Ϊ���� | |
| D�� | ����Ϊ����Ⱦ��ˮ�����ڻ����Ѻõ�� |
19��������ԭ��Ӧ�����ֻ������ͷ�Ӧ�Ĺ�ϵ��ͼ��ʾ�������л�ѧ��Ӧ��������3�ģ�������
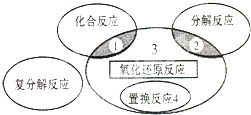

| A�� | 4Fe��0H��2+O2+2H2O�T4Fe��OH��3 | B�� | 2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+H2 O+CO2�� | ||
| C�� | 4NH3+5O2 $\frac{\underline{����}}{��}$4NO+6H2O | D�� | Zn+H2SO4�TZnSO4+H2�� |
16��������������ͬ���칹����ǣ�������
| A�� | ������������ | B�� | ��������ѿ�� | C�� | �����ͺ�֬�� | D�� | ���ۺ���ά�� |
17������п�̸ɵ����һ��һ�ε�أ����Ϊ����п���м���̼��������Χ����̼�ۣ��������̣��Ȼ�п���Ȼ�淋���ɵ������õ���ڷŵ���̲���MnOOH�����մ����÷ϵ�ؿ��Եõ����ֻ���ԭ�ϣ��й����ݱ���ʾ��
�ܽ��/��g/100gˮ��
�ش��������⣺
��1���õ�صĸ���Ϊп��������ӦʽΪMnO2+H++e-=MnOOH��
��2��ά�ֵ���ǿ��Ϊ0.5A����ع���5���ӣ���������Zn0.05gg������֪F=96500C/mol��
��3���ϵ�غ�״������ˮ�������ˣ���Һ����Ҫ���Ȼ�п���Ȼ�泥����߿���ͨ������Ũ������ȴ�ᾧ������գ���������Ҫ�ɷ��Ƕ������̡�̼�ۺ�MnOOH�������еõ��ϴ��Ķ������̣�����ķ����ǿ����м��ȣ���ԭ����̼��ת��Ϊ������̼��MnOOH����Ϊ�������̣�
��4���÷ϵ�ص�пƤ������ˮ������п����ȥ���������������䷽���ǣ�����ϡ�����˫��ˮ���ܽ⣬����ΪFe3+�Ӽ����PHΪ2.7�����պ���ȫ����������Ũ�ȡ�1��10-5 mo1•L-1ʱ������Ϊ���ӳ�����ȫ�������Ӽ����PHΪ6п��ʼ�������ٶ���ʱ��Һ��Zn2+��Ũ��Ϊ0.1mo1•L-1�������������̲���˫��ˮ�ĺ��Zn2+��Fe2+���벻����ԭ����Fe��OH��2��Zn��OH��2��Ksp�����
�ܽ��/��g/100gˮ��
�¶�/�� ������ | 0 | 20 | 40 | 60 | 80 | 100 |
| NH4Cl | 29.3 | 37.2 | 45.8 | 55.3 | 65.6 | 77.3 |
| ZnCl2 | 343 | 395 | 452 | 488 | 541 | 614 |
| ������ | Zn��OH��2 | Fe��OH��2 | Fe��OH��3 |
| Ksp����ֵ | 10-17 | 10-17 | 10-39 |
��1���õ�صĸ���Ϊп��������ӦʽΪMnO2+H++e-=MnOOH��
��2��ά�ֵ���ǿ��Ϊ0.5A����ع���5���ӣ���������Zn0.05gg������֪F=96500C/mol��
��3���ϵ�غ�״������ˮ�������ˣ���Һ����Ҫ���Ȼ�п���Ȼ�泥����߿���ͨ������Ũ������ȴ�ᾧ������գ���������Ҫ�ɷ��Ƕ������̡�̼�ۺ�MnOOH�������еõ��ϴ��Ķ������̣�����ķ����ǿ����м��ȣ���ԭ����̼��ת��Ϊ������̼��MnOOH����Ϊ�������̣�
��4���÷ϵ�ص�пƤ������ˮ������п����ȥ���������������䷽���ǣ�����ϡ�����˫��ˮ���ܽ⣬����ΪFe3+�Ӽ����PHΪ2.7�����պ���ȫ����������Ũ�ȡ�1��10-5 mo1•L-1ʱ������Ϊ���ӳ�����ȫ�������Ӽ����PHΪ6п��ʼ�������ٶ���ʱ��Һ��Zn2+��Ũ��Ϊ0.1mo1•L-1�������������̲���˫��ˮ�ĺ��Zn2+��Fe2+���벻����ԭ����Fe��OH��2��Zn��OH��2��Ksp�����
 Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���