��Ŀ����
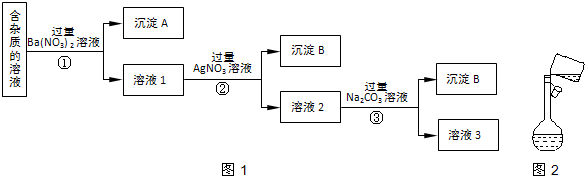
6������������һ�ֳ�����ʳƷ���Ӽ���Ϊ����ijʳƷ���������κ�����ͨ����1kg��Ʒ�к�SO2�������ƣ���ij�о�С��ͬѧ���������ʵ�����̣������Լ���Ϊ��������
��ش�
��1������������ΪʳƷ���Ӽ���������a������ĸ����
a���������� b����ǿӪ��
��2����Ӧ����ͨ��N2��Ŀ����c������ĸ����
a����ȴ����������
b�������ɵ����巴Ӧ
c�������ɵ�����ȫ���ϳ�
��3����Ӧ�١���������������ԭ��Ӧ���Ǣڣ�����ţ���
��4����ȡ��ƷX g����Ӧ������0.01mol/L NaOH��Һ100mL����1kg��Ʒ�к�SO2��������$\frac{32}{X}$ g���ú�X�Ĵ���ʽ��ʾ����
���� ��1������������Ӿ���ǿ�Ļ�ԭ�ԣ��ױ���������Ϊ�����Σ�
��2������������ӿ��Ժ�ǿ��֮�䷴Ӧ�õ������������Ͷ�������֮�䲻�ᷴӦ��
��3����Ԫ�ػ��ϼ۱仯�ķ�Ӧ��������ԭ��Ӧ��
��4�����ݻ�ѧ��Ӧ���ԭ���غ�֪ʶ���ش��жϣ�
��� �⣺����������Ʒ�м���ϡ����֮�ᷢ����Ӧ�õ������������壬��������ᱻ������ȫ�ų����õ�����A�Ƕ�������͵���������������л�ԭ�ԣ��ܱ�˫��ˮ����Ϊ���ᣬ������Ժ���������֮�䷢���кͷ�Ӧ���������ƣ�
��1������������Ӿ���ǿ�Ļ�ԭ�ԣ��ױ���������Ϊ�ȶ��������Σ������������ο��Է������ʣ���ΪʳƷ���Ӽ����ʴ�Ϊ��a��
��2������������Ʒ�м���ϡ����֮�ᷢ����Ӧ�õ������������壬�����Ͷ�������֮�䲻�ᷴӦ����Ӧ����ͨ��N2���Խ����ɵ������������ȫ���ϳ���
�ʴ�Ϊ��c��
��3����Ӧ��������������Ӻ�������֮��ķ�Ӧ������������ԭ��Ӧ�����Ƕ��������˫��ˮ֮��ķ�Ӧ������������ԭ��Ӧ�����������������֮����кͷ�Ӧ��������������ԭ��Ӧ���ʴ�Ϊ���ڣ�
��4��������0.01mol/L NaOH��Һ100mL��������������ʵ�����0.0005mol��������ԭ�ӵ����ʵ�����0.0005mol��������ԭ���غ㣬Xg��Ʒ�к��еĶ��������������
0.0005mol��64g/mol=0.032g����1kg��Ʒ�к�SO2��������$\frac{32}{X}$g���ʴ�Ϊ��$\frac{32}{X}$��
���� �����Թ���������ķ�ʽ����Ԫ���Լ������������֪ʶ��ע��ԭ���غ�˼���ڽ����е�Ӧ�ã����鿼���Ի�ѧʵ�鷽���ķ���������������������Ŀ�Ѷ��еȣ�

| A�� | ���Ȼ�̼�ĵ���ʽ��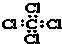 | B�� | CH4���ӵı���ģ�ͣ� | ||
| C�� | ��ϩ�����ģ�ͣ�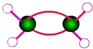 | D�� | ��Ȳ�Ľṹ��ʽ��CH��CH |
| A�� | ʹ�ô�����ʹ��ѧ��Ӧ���ʼӿ죬ƽ�ⳣ������ | |
| B�� | ƽ�ⳣ�����¶ȵĸı���ı� | |
| C�� | ��ѧƽ�ⷢ���ƶ���ƽ�ⳣ���ط����仯 | |
| D�� | ����3Fe��s��+4H2O��g��?Fe3O4��s��+4H2��g������Ӧ�Ļ�ѧƽ�ⳣ���ı���ʽΪK=$\frac{c��F{e}_{3}{O}_{4}��•{c}^{4}��{H}_{2}��}{c��Fe��•{c}^{4}��{H}_{2}O��}$ |
| A�� | �ù�ҵ���������̲� | B�� | ��ţ������������ | ||
| C�� | �ö�������Ѭ������ | D�� | �ô������ʳ |
| A�� | c��HX����c��X-�� | B�� | c��HX��+c��H+��=c��Na+��+c��OH-�� | ||
| C�� | c��X-��+c��HX��=2c��Na+�� | D�� | c��OH-����c��H+�� |
| A�� | ����Ӧ��Ũ�� | B�� | �Ӵ��� | C�� | ��ѹ | D�� | ���� |