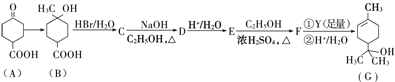��Ŀ����
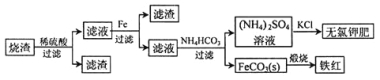
����Ŀ�����������յõ�����������Ҫ�ɷ�ΪFeO��Fe3O4��Fe2O3�ȡ����ø������Ʊ��������Ʒ���ȼطʵ��������£�
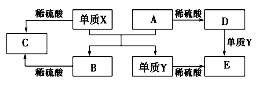
�ش��������⣺
��1������������������Fe2O3��SO2�������ɱ�״����11.2L SO2���壬ת�Ƶ��ӵ����ʵ���Ϊ____________��
��2���������м������۵�������____________�������ӷ���ʽ��ʾ����
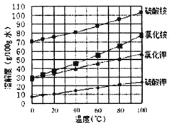
��3����֪�����ε��ܽ�����¶ȱ仯��������ͼ��ʾ����ش��������⣺
������ҺII�м���NH4HCO3��Һ��������Ӧ�����ӷ���ʽ��________________��
����(NH4)2SO4��Һ����KCl��õ����ȼط�Ӧ���еIJ���Ϊ_________��________��ϴ�ӡ�����ȣ���Ӧ�Ļ�ѧ����ʽΪ_______________��
��4���ú���������������������������ͭ��Һ�Ʊ�CuSO4��5H2O���������£�
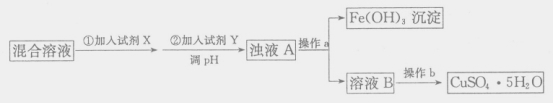
�����Լ�X��Ŀ����_____��������ҺpH���Լ�Y������_____������ĸ��ţ���
a��NaOH
b��CuO
c��NH3��H2O
d��Cu��OH��2CO3
Cu2+Ϊ0.2mol��L-1����Һ��������Fe3+��������Ũ��С��1*10-5mol��L-1ʱ�������ѳ�����ȫ����������ҺpH�ľ�ȷ��Χ��_____����֪��Kap��Cu��OH��2��=2��10-20��Kap��Fe��OH��3��=8.0��10-38��lg2=0.3����
���𰸡���1��3.5mol��2��Fe+2Fe3+=3Fe2+��3����Fe2++2HCO3-=FeCO3��+CO2��+H2O
�������ᾧ���ȹ�����NH4��2SO4+2KCl![]() K2SO4��+2NH4Cl
K2SO4��+2NH4Cl
��4����Fe2+����ΪFe3+bd3.3��pH<4.5
��������
�����������1����������������Fe2O3��SO2����Ӧ��1molFeSʧȥ7mol���ӣ�ͬʱ����1molSO2���������ɱ�״����11.2L SO2������0.5molSO2ʱ��ת�Ƶ��ӵ����ʵ���Ϊ![]() ��
��
��2����Һ�к��������ӣ�����Ҫת��Ϊ̼�������������������м������۵���������ԭ�����ӣ���Ӧ�������ӷ���ʽΪFe+2Fe3+=3Fe2+��
��3������������ͼ��֪����ҺII�м���NH4HCO3��Һ����̼������������ԭ���غ��֪��Ӧ�л���CO2��ˮ���ɣ�����������Ӧ�����ӷ���ʽ��Fe2++2HCO3-=FeCO3��+CO2��+H2O��
�������ܽ�����߿�֪�Ȼ�淋��ܽ�����¶ȵ�Ӱ���������أ�������(NH4)2SO4��Һ����KCl��õ����ȼط�Ӧ���еIJ���Ϊ�����ᾧ�����ȹ�����ϴ�ӡ����������Ӧ�Ļ�ѧ����ʽΪ��NH4��2SO4+2KCl![]() K2SO4��+2NH4Cl��
K2SO4��+2NH4Cl��
��4����������ͼ���������Լ�X��Ŀ��������Һ�е���������ת��Ϊ�����ӣ��Ӷ�����ת��Ϊ�������������������Ʊ�����ʱ�������������ʣ���������ҺpH���Լ�Y����������ͭ���ʽ̼��ͭ����ѡbd�����������������ܽ�ȳ�����֪����������ȫ����ʱ��Һ����������Ũ����![]() ������pH��3.3��ͭ���ӿ�ʼ����ʱ��Һ��������Ũ����
������pH��3.3��ͭ���ӿ�ʼ����ʱ��Һ��������Ũ����![]() ������pH��4.5�����������ҺpH�ľ�ȷ��Χ��3.3��pH<4.5��
������pH��4.5�����������ҺpH�ľ�ȷ��Χ��3.3��pH<4.5��

 ��У����ϵ�д�
��У����ϵ�д�