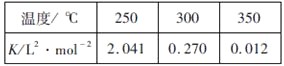
��Ŀ����
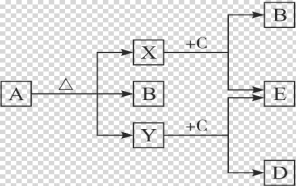
����Ŀ����֪��A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·������ͼ��ʾ��
�ش��������⣺
��1��д��A�ĵ���ʽ ����ռ乹�� ��
��2��B��D�����еĹ��������Ʒֱ��� �� ��
��3��д�����з�Ӧ�ķ�Ӧ���ͣ��� �� ��
��4��д��A��B��Ӧ�Ļ�ѧ����ʽ�� ��
��5��д��B��D��Ӧ�������������Ļ�ѧ��Ӧ����ʽ�� ��
���𰸡���1��![]() �� ƽ����
�� ƽ����
��2���ǻ����Ȼ� ��
��3��������ȡ��(����)��
��4��CH2=CH2��H2O��CH3CH2OH(����������) ��
��5��CH3CH 2OH��CH3COOH��CH3COOCH2CH3��H2O(������Ũ���ᣬ����)��
��������
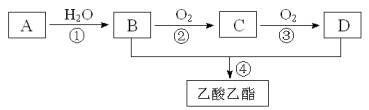
������������������Ϣ�ƶ�AΪCH2=CH2��BΪCH3CH2OH��CΪCH3CHO��DΪCH3COOH��
��1��AΪCH2=CH2������ʽΪ![]() ����ռ乹��Ϊƽ������
����ռ乹��Ϊƽ������
��2��BΪCH3CH2OH��DΪCH3COOH�������еĹ��������Ʒֱ����ǻ����Ȼ���
��3����Ӧ�����Ҵ���������Ӧ������ȩ����Ӧ����Ϊ������Ӧ����Ӧ����������Ҵ���Ӧ��������������ˮ����Ӧ����Ϊȡ����Ӧ��������Ӧ��
��4����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�����ѧ����ʽΪCH2=CH2��H2O![]() CH3CH2OH��
CH3CH2OH��
��5���Ҵ������ᷴӦ��������������ˮ����ѧ��Ӧ����ʽΪ
CH3CH 2OH��CH3COOH![]() CH3COOCH2CH3��H2O��
CH3COOCH2CH3��H2O��