��Ŀ����
����Ŀ����1��K2Cr2O7 + 14HCl= 2KCl + 2CrCl3 + 3Cl2��+ 7H2O ���á������š���ʾ����ת�Ƶķ������Ŀ��___,���������뻹ԭ��������ʵ���֮��Ϊ_____________��
��2��______mol H2O�й�����9.03��1022��ԭ�ӣ�������Ϊ_______��
��3����ƽ����������ԭ��Ӧ����ʽ��___KMnO4+___H2S+__H2SO4(ϡ) ��__MnSO4+__S��+__K2SO4+__H2O
��4��Cl2��һ���ж����壬���й©��������صĻ�����Ⱦ������������Ũ��ˮ������Cl2�Ƿ�й©���йط�Ӧ�Ļ�ѧ����ʽΪ��3Cl2��������8NH3��������6NH4Cl���̣���N2������������Ӧ������Cl2 1.5 mol, ��������NH3�ڱ�״���µ����Ϊ______ L��
���𰸡�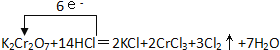 3:2 0.05 0.9 2 5 3 2 5 1 8 22.4
3:2 0.05 0.9 2 5 3 2 5 1 8 22.4
��������
��1�����ݷ���ʽȷ��ת�Ƶ�����Ŀ�����������ṩ���ӵ�����(Ԫ��)ָ��õ��ӵ�����(Ԫ��)���ݻ��ϼ۱仯�жϻ�ԭ�������������ݷ���ʽ�ж����������뻹ԭ��������ʵ���֮�ȣ�
��2������n=N/NA����9.03��1022��ԭ�ӵ����ʵ��������1��ˮ���Ӻ���ԭ����Ŀ����ˮ�����ʵ���������m=nM����ˮ��������
��3������Ԫ�صĻ��ϼ۱仯������õ��ӵ�ʧ�غ��Լ�ԭ���غ���ƽ��
��4���������İ������ɵ��������ݷ���ʽ�������������ʵ����������ɵ��������ʵ������ٸ��ݵ�ԭ���غ���㱻�����İ��������ʵ���������V=nVm���㱻�����İ����������
��1����Ӧ��CrԪ�ػ��ϼ۽��ͣ�Ԫ�ػ��ϼ���+6�۽���Ϊ+3�ۣ���Ԫ�ػ��ϼ۴ӣ�1�����ߵ�0�ۣ���Ӧ�й�ת�Ƶ�����Ϊ6e-���õ����ŷ��������ת�Ƶķ������Ŀ�ɱ�ʾΪ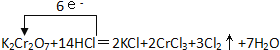 ��K2Cr2O7������CrԪ�ػ��ϼ۽��ͣ�K2Cr2O7Ϊ�����������ݻ��ϼ۱仯��֪��CrCl3�ǻ�ԭ���Cl2������������ݷ���ʽ��֪���������뻹ԭ��������ʵ���֮��Ϊ3��2��
��K2Cr2O7������CrԪ�ػ��ϼ۽��ͣ�K2Cr2O7Ϊ�����������ݻ��ϼ۱仯��֪��CrCl3�ǻ�ԭ���Cl2������������ݷ���ʽ��֪���������뻹ԭ��������ʵ���֮��Ϊ3��2��
��2��9.03��1022��ԭ�ӵ����ʵ���Ϊ![]() =0.15mol��1��ˮ���Ӻ���3��ԭ�ӣ���ˮ�����ʵ���Ϊ0.15mol��3=0.05mol����ˮ������Ϊ0.05mol��18g/mol=0.9g��
=0.15mol��1��ˮ���Ӻ���3��ԭ�ӣ���ˮ�����ʵ���Ϊ0.15mol��3=0.05mol����ˮ������Ϊ0.05mol��18g/mol=0.9g��
��3����Ӧ��KMnO4��MnSO4��MnԪ�ػ��ϼ���+7����Ϊ+2��������5�ۣ�H2S��S��SԪ�ػ��ϼ���-2������Ϊ0�ۣ�������Ϊ2�ۣ����ϼ�������С������Ϊ10����KMnO4��ϵ��Ϊ2��H2S��ϵ��Ϊ5���ٸ���ԭ���غ���ƽ�������ʵ�ϵ������ƽ��ʽΪ��2KMnO4+5H2S+3H2SO4(ϡ)��2MnSO4+5S��+K2SO4+8H2O��
��4���������İ������ɵ��������ݷ���ʽ��֪���ɰ��������ʵ���Ϊ1.5mol��3=0.5mol�����ݵ�ԭ���غ��֪�������İ��������ʵ���Ϊ0.5mol��2=1mol���ʱ������İ��������Ϊ1mol��22.4L/mol=22.4L��